
| ѡ�� | ���� | ���鷽�� |
| A | CO32- | ����Ʒ�м����������ᣬ�����ɵ���ɫ��ζ������ͨ�����ʯ��ˮ�У��۲���Һ�Ƿ����� |
| B | SO42- | ����Ʒ���ȼ���ϡ�����ữ���ٵμ��Ȼ�����Һ���۲��Ƿ��а�ɫ�������� |
| C | Fe2+ | ȡ������Һ���Թ��У��������Ը��������Һ���۲���Һ��ɫ�Ƿ���ȥ |
| D | I- | ȡ������Һ���Թ��У�����������ˮ���ټ��������Һ���۲���Һ�Ƿ����ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��ԭ��Һ�п��ܺ���̼��������ӣ���һ������̼������ӣ�
B��Ӧ����ϡ�����ữ������ԭ��Һ������������������ӣ��ᱻϡ������������������ӣ�
C��ԭ��Һ�к��к�������������ӣ���һ�������������ӣ�
D�����ݵⵥ���ܹ�ʹ���۱��������Խ��м���
��� �⣺A������Ʒ�м����������ᣬ�����ɵ���ɫ��ζ������ͨ�����ʯ��ˮ�У��۲���Һ�Ƿ����ǣ�������Ϊ������̼��ԭ��Һ�п��ܺ���̼��������ӣ���һ������CO32-����A����
B������Ʒ���ȼ���ϡ�����ữ��ϡ�����ܹ���������������ӣ���ԭ��Һ�п��ܺ�������������ӣ�Ӧ����ϡ�����ữ����B����
C��ȡ������Һ���Թ��У��������Ը��������Һ����Һ��ɫ��ֻ��˵��ԭ��Һ�к��л�ԭ�����ӣ�����Ϊ����������ӣ���һ������Fe2+����C����
D���ⵥ���ܹ�ʹ������Һ���������Լ�������ӵķ���Ϊ��ȡ������Һ���Թ��У�����������ˮ���ټ��������Һ���۲���Һ�Ƿ����ɫ����D��ȷ��
��ѡD��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ע��������ӵĴ������ʱ����Ҫ�ų��������ӣ�ȷ�����鷽���������ԣ�


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ҫ���ʵ�����ȡ10.6gNa2CO3•10H20���� | |
| B�� | ��ʵ�����õ�������ֻ��ҩ�ס����������ձ�����ͷ�ιܺ�100 mL����ƿ | |
| C�� | ����ʱ������ƿ�����������������ˮ�ᵼ��Ũ��ƫ�� | |
| D�� | ����ʱ�ý�ͷ�ιܵ��˵�����ˮ��������ƿ�ڲ��̶����Ϸ��ᵼ��Ũ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1L pH=1��HCl��Һ�У�H+����Ϊ0.1NA | |
| B�� | 8.4gNaHCO3����ˮ����Һ�к���0.1NA��CO32- | |
| C�� | ��״���£�11.2 L���Ȼ�̼�к��е�C-Cl������ĿΪ2NA | |
| D�� | ��⾫��ͭʱ����������������ÿ����64g��������·ͨ��������Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
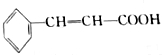 Ҳ���ڷ�ʽ�ṹ��1mol
Ҳ���ڷ�ʽ�ṹ��1mol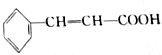 ��������4molH2�����ӳɷ�Ӧ��
��������4molH2�����ӳɷ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
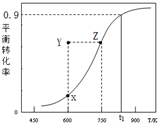 �״��������ȡ��ȩCH3OH��g��?HCHO��g��+H2��g�����״���ƽ��ת�������¶ȱ仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������
�״��������ȡ��ȩCH3OH��g��?HCHO��g��+H2��g�����״���ƽ��ת�������¶ȱ仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | ���ⷴӦ�ġ�H��0 | B�� | ��t1Kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ8.1 | ||
| C�� | 600Kʱ��Y��״��Ħԣ��������ԣ��棩 | D�� | ��������ϵѹǿ�����߽�����ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
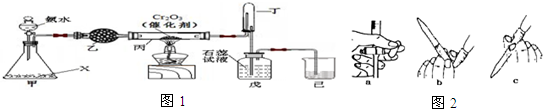
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MgƬ���������缫��Ӧ��Mg-2e-�TMg2+ | |
| B�� | AlƬ���������缫��Ӧ��Al+4OH--3e-�TAlO2-+2H2O | |
| C�� | ���Ӵ�Mg�缫�ص�������Al�缫 | |
| D�� | AlƬ�������ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=1����Һ�У�Fe2+��NO${\;}_{3}^{-}$��SO${\;}_{4}^{2-}$��Cl- | |
| B�� | ��ˮ�������c��OH-��=1��10-13 mol•L-1����Һ�У�Na+��AlO${\;}_{2}^{-}$��S2-��CO${\;}_{3}^{2-}$ | |
| C�� | ���д���Fe3+����Һ�У�Na+��SCN-��K+��NO${\;}_{3}^{-}$ | |
| D�� | c��H+��=10-14 mol•L-1����Һ�У�Mg2+��NO${\;}_{3}^{-}$��Fe2+��ClO- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com