
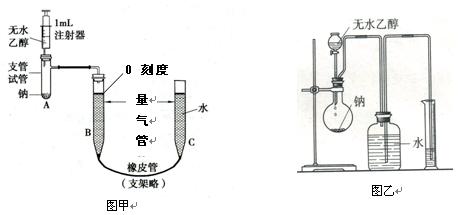
 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
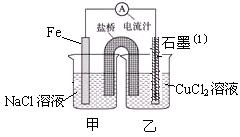
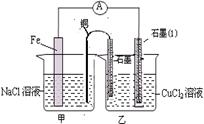
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
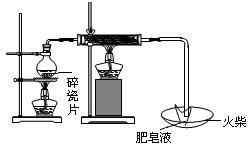
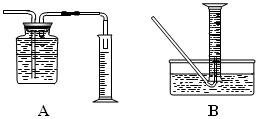
| | ���� | ��������� |
| ���ܢ� | | |
| ���ܢ� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
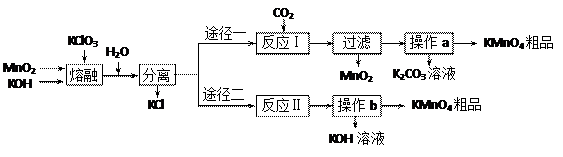 ��֪����3MnO2+KClO3+6KOH
��֪����3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O| �¶� | �ܽ��/g | ||
| K2CO3 | KOH | KMnO4 | |
| 20�� | 111 | 112 | 6.38 |
| 60�� | 127 | 154 | 22.1 |
| A�������� | B���ձ� | C���ƾ��� | D�������� |
 �Ʒ�Ӧ�������������뻹ԭ��������ʵ���֮��Ϊ �� ���÷�Ӧ�п���ѭ�����õIJ����� �� ��
�Ʒ�Ӧ�������������뻹ԭ��������ʵ���֮��Ϊ �� ���÷�Ӧ�п���ѭ�����õIJ����� �� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ձ���� | �� | �� | �� |
| �����Լ� | ����0.1g | ����0.1g��0.1mol/LH2SO43mL | 0.1mol/LH2SO43mL |
| ��ɫ����ʱ��(��) | 1�� | 4������ | 8������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

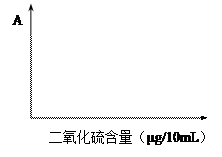
| �� �� | 0 | 1 | 2 | 3 | 4 | 5 |
| ��Һ(ml) | 0 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
| ����Һ(ml) | 10.00 | 9.80 | 9.60 | 9.40 | 9.20 | 9.00 |
| ����������(��g) | 0 | 5.00 | 10.00 | 15.00 | 20.00 | 25.00 |
| �� �� | 0 | 1 | 2 | 3 | 4 | 5 |
| ����� | 0 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ��û��Na2CO3��Ba(NO3)2 |
| B��һ����Na2CO3��û��Ba(NO3)2 |
| C��û��Ba��NO3��2����KNO3�����ܻ���Na2CO3 |
| D��û��Na2CO3����Ba(NO3)2��KNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com