
���� I����1��ʳ�����ʵ���Ũ��Ϊ0.7mol•L-1��������Ϊ0.75mol•L-1��60g/mol=45g/L��
��2��������̼��Ʒ�Ӧ���ɴ���ơ�ˮ�Ͷ�����̼��
II����1����������һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ��ѡ����ʵ�������
��2�����Ũ��������ʵ���Ũ��c=$\frac{1000�Ѧ�}{M}$��Ȼ�����ϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
��3������c=$\frac{n}{V}$��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
III����1����Һ��ͨ��һ������û��������������ͨ��һ�����߳��ֹ�����·��Ϊ���������
��2���������������Һ�����۳���
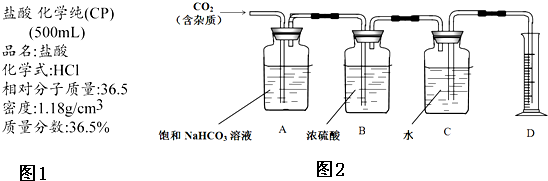
IV����1��CO2����ˮ���ռ������ռ�֮ǰ����Ҫ��������и��
��2�������ӷ������ɵ�CO2�����к��лӷ������Ȼ������壬Aװ���еı���NaHCO3��Һ���������Ȼ��⣻
��3��ʵ��ǰ���Cװ�ã���ˮ������Ϊ50.00g��ʵ����Ϻ�Cװ�ã���ˮ������Ϊ40.02g��D����Ͳ����Ϊ10.0 mL����˵���ռ���CO2������=40.02g+10.0g-50.0g=0.02g���������10.0ml������CO2���ܶ�=$\frac{0.02g}{0.01L}$=2g/L����֪H2�ܶ�Ϊ0.09g/L���������ݾ����ۺ�Ϊ�������ֵ�������������ͬ������������ܶ�֮�ȵ�����Է�������֮�ȿ�֪�ɼ����������̼����Է���������
��� �⣺I����1��ʳ�����ʵ���Ũ��Ϊ0.75mol•L-1��������Ϊ0.75mol•L-1��60g/mol=45g/L����4.50g/100mL��Ϊ����ף�
�ʴ�Ϊ��4.50�����죻
��2��������̼��Ʒ�Ӧ���ɴ���ơ�ˮ�Ͷ�����̼�����ӷ���ʽ��2CH3COOH+CaCO3=2CH3COO-+CO2��+H2O+Ca2+��
�ʴ�Ϊ��2CH3COOH+CaCO3=2CH3COO-+CO2��+H2O+Ca2+��
II����1����������һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȿ�֪��Ҫ�������У���Ͳ���ձ�����������100mL����ƿ�ͽ�ͷ�ιܣ�����Ҫѡ�õ������Т٢ۢݢߣ���ȱ�ٵ�������100mL����ƿ���ʴ�Ϊ���٢ۢݢߣ�100mL����ƿ��
��2��Ũ��������ʵ���Ũ��c=$\frac{1000��1.18��36.5%}{36.5}$mol/L=11.8mol/L������Ҫ��Ũ��������ΪVmL��Ȼ�����ϡ�Ͷ���cŨVŨ=cϡVϡ��֪��
11.8mol/L��VmL=100mL��1mol•L-1�����V=8.5mL���ʴ�Ϊ��8.5��
��3��A������ʱ��������ƿ�̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���A����
B������ƿ��ʹ��ǰδ�����������������ˮ����Ũ����Ӱ�죬��B����
C��ת����Һ��δϴ���ձ��Ͳ��������ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ���C��ȷ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ��ټ�ˮ���̶��ߣ�����Һ������ӣ�����Ũ��ƫС����D��ȷ��
�ʴ�Ϊ��CD��
III����1�������ܲ��������ЧӦ�������ĵ������Ƴ���Һ���ڽ��壬����ü����������Һ�����Է���һ������������������Һ���������Ϊ�����ЧӦ��
�ʴ�Ϊ�������ЧӦ��
��2������͵���ʻ�Ͽ��Ծ۳��������ǵ�����Ȼ����ˮ��Һ�����Խ����ƺõ�������Һ���뵽��������Һ�У��������״�������������Ϊ����ľ۳���
�ʴ�Ϊ������ľ۳���
IV����1������װ�ÿ�֪��CO2����ˮ���ռ���������ռ�֮ǰ����Ҫ��������и����û�б�Ҫ���Bװ�ã�
�ʴ�Ϊ���ޣ���ˮ���ռ����壬����Ҫ��������и��
��2�����������ӷ���������ɵ�CO2�����к��лӷ������Ȼ������壬����Aװ���еı���NaHCO3��Һ�������������Ȼ��⣬
�ʴ�Ϊ��HCl��
��3��ʵ��ǰ���Cװ�ã���ˮ������Ϊ50.00g��ʵ����Ϻ�Cװ�ã���ˮ������Ϊ40.02g��D����Ͳ����Ϊ10.0 mL����˵���ռ���CO2������=40.02g+10.0g-50.0g=0.02g���������10.0ml������CO2���ܶ�Ϊ��$\frac{0.02g}{0.01L}$=2g/L��
��֪H2�ܶ�Ϊ0.09g/L���������ݾ����ۺ�Ϊ�������ֵ����
����ͬ������������ܶ�֮�ȵ�����Է�������֮�ȣ�
����CO2����Է�������Ϊ��$\frac{2}{0.09}$��2=44.4��
�ʴ�Ϊ��44.4��
���� ���⿼��������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ��漰����һ�����ʵ���Ũ�ȵ���Һ����������������ѧ���㡢��������ʡ����ӷ���ʽ��д��֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ�����������������ѧʵ�顢���м���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������֯�з�����������ʱ������ת��Ϊ��ѧ�� | |
| B�� | ��ɫֲ����й������ʱ��̫����ת��Ϊ������ | |
| C�� | úȼ��ʱ����ѧ����Ҫת��Ϊ���� | |
| D�� | ���ˮ��������������ʱ������ת��Ϊ��ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼ˮ������ | B�� | ���� | C�� | ���� | D�� | ̼�⻯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ���� | B�� | ��оƬ | C�� | ��� | D�� | ��ͨˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
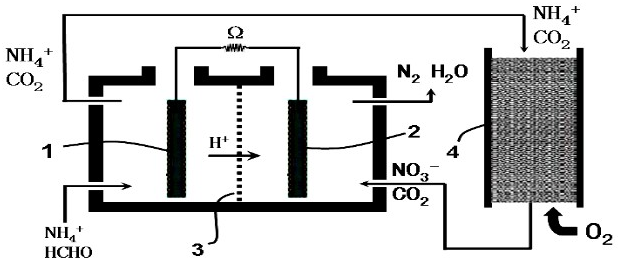
| A�� | ���������ķ�ӦΪ��HCHO-4e-+H2O�TCO2+4H+ | |
| B�� | �������ڵ��������豣���������� | |
| C�� | NH4+ͨ��ѭ���������ձ�ת����N2 | |
| D�� | O2����������Ӧ��������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������֡��ơ�ʳ�� | B�� | ���͡���֬����ȩ��֬ | ||
| C�� | ʯ̿�ᡢ���ᡢ������ | D�� | ���͡��Ҵ��ơ��ȷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
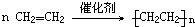 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com