
��ˮAlCl3��һ����Ҫ���л��ϳɴ�������������183��ʱ����������ʪ��������������������ij��ѧ��ѧ��ȤС����������ѧ�����������ʵ���Ʊ���ˮAlCl3��ʵ��װ������ͼ��ʾ��
��ش��������⣺
��1���Ʊ�ʵ�鿪ʼʱ���ȼ��װ�õ������ԣ��������IJ��������� ��
a������MnO2��ĩ b����ȼA�оƾ��� c������Ũ���� d����ȼD���ƾ���
��2��д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3��װ��B��C�е��Լ��ֱ��� ��
��4����ͬѧ��ΪF��G������һ������������Ҽ���һ��ҩƷ���ɴﵽ��ͬЧ��������ҩƷ������ ��
��5��E�еõ�������ɫ��ĩ������ľ��������Թ۲쵽��ƿ���а������ɣ��û�ѧ����ʽ��ʾ��ԭ�� ��
��6���Ʊ����������������Ũ���½�����������ȡ��Ӧ��ֹͣ��Ϊ�ⶨ����Һ�������Ũ�ȣ�ijͬѧ��ȡ����Һ10.00mL����ˮϡ�͵�250.00mL��Ȼ�����ȡ��20.00mL����0.1000mol��L-1��NaOH����Һ���еζ����յ�ʱ����NaOH��Һ24.00mL����ò���Һ���������Ũ��Ϊ ��
��ÿ��2�֣���12�֣�
��1�� acbd��2��MnO2 + 4HCl(Ũ) MnCl2+ Cl2��+ 2H2O
MnCl2+ Cl2��+ 2H2O
��3������ʳ��ˮ��ˮ����Ũ���� ��4)��ʯ�һ�������
��5��AlCl3+3H2O= Al(OH)3+3HCl
��6��3.00 mol/L
���������������1��װ�������Լ������Ժ�Ҫ���ȼ������������̣�Ȼ�����Ũ���ᡣ���ڽ������ǻ��õĽ��������ױ��������������ȵ�ȼA���ƾ��ƣ�Ȼ���ȼD���ƾ��ƣ�����ȷ�IJ���˳����acbd��
��2��Aװ�����Ʊ������ģ���Aװ���з�����Ӧ�Ļ�ѧ����ʽMnO2 + 4HCl(Ũ) MnCl2+ Cl2��+ 2H2O��
MnCl2+ Cl2��+ 2H2O��
��3���������ɵ������к����Ȼ����ˮ������������Ȼ������Ʊ�����Ӧ��ȥ����B��ʢ�ű���ʳ��ˮ��������ȥ�Ȼ��⣬C��ʢ��Ũ���ᣬ��������������
��4����Ϊ�Ȼ�������ˮ�⣬��Ӧ�÷�ֹ�����е�ˮ��������Eװ�ã���G�����տ����е�CO2�����Կ��Լ����ʯ��������F��G�����á�
��5�������Ȼ�������ˮ�����������������Ȼ��⣬���Ȼ�����Һ�γɰ�������Ӧ�Ļ�ѧ����ʽ��AlCl3+3H2O�� Al(OH)3+3HCl��
��6�����ݷ���ʽHCl��NaOH=NaCl��H2O��֪�������Ũ����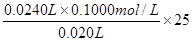 ��3.00mol/L��
��3.00mol/L��
���㣺�����������Ȼ������Ʊ�������ij��ӡ��������к͵ζ��ļ����


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����������壺H2��Cl2��CH4��HCl��NH3��NO��H2S��SO2������ͼװ�ý���ʵ�飬��д���пհף�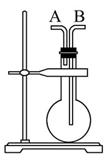
(1)����ƿ����ʱ����A�ڽ������ռ��������ǣߣߣߣߣߣ���B�ڽ������ռ��������ǣߣߣߣߡ�
(2)����ƿ�г���ˮʱ�������������ߣߣߣߵ�����������
(3)����ƿ��װ��ij����Һ������ϴ��ʱ������Ӧ�ӣߣߣߣ߿ڽ�����ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���⣺I�����з���������ʵ�������Ʊ���������____________________
A����̬�Ȼ�識��ȷֽ� B����������������еμ�Ũ��ˮ
C���Ȼ����Һ������������Һ���� D�������Ȼ�����������ƹ����ϼ���
II��Ϊ����ʵ���������ù�ҵԭ���Ʊ����������������������ͼ��ʾ��װ�ã�ͼ�мг�װ�þ�����ȥ����
ʵ��������£�
�ټ��װ�õ������Ժرյ��ɼ�A��B��C��D��e����A�м���п������©��ע��һ������ϡ���ᡣ���ɼ�C��D��e����A����������������F���ڴ��ռ��������������䴿�ȡ�
�ڹرյ��ɼ�c��ȡ�½�ȥ�ײ���ϸ��ƿC�����ɼ�a��������������B�鴿���ȼ,Ȼ������������ϸ��ƿC������ƿ������ͼ��ʾ������������ƿ��ȼ�գ������Ӻ����Ϩ��
���þƾ��Ƽ��ȷ�Ӧ��E������ͨ������������ϸ��ƿC��ˮλ�½���Һ�汣�ֲ���ʱ�����ɼ�b����ϸ��ƿC�����徭D���뷴Ӧ��E��Ƭ�̺�F�е���Һ��졣
��ش��������⣺
(1)д����Ӧ��E�з�����Ӧ�Ļ�ѧ����ʽ_______________________________________
�÷���ʽ����F����Һ����ԭ��______________________________________________
(2)Cƿ��ˮλ�½���Һ�汣�ֲ���ʱ��Aװ���ڷ���������Ϊ____________________________����ֹ��ʵ��װ����ѹǿ����ʱ�ٴ��ɼ�b��ԭ����_____________________________________,��C���������Ҫ�ɷ�Ϊ��__________________________________________.
(3)Ϊʲô�þƾ��Ƽ��ȷ�Ӧ��E-��ʱ����ٴ��ɼ�b____________________________
(4)ΪʲôFװ�ò��÷�������װ��_________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
CO���Ʊ�ԭ����HCOOH CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��
CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��
����д���пհף�
��1��ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2-����������ѡ����ʵ��Լ��Ʊ���������ϩ��д���Ʊ���ϩ�Ļ�ѧ����ʽ�� ��
��2����������װ���Ʊ����﴿����CO��װ����a�������� ��װ����b�������� ��c��ʢװ���Լ��� ��
��3���Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳���� ������ţ�
�ٱ���Na2SO3��Һ��������KMnO4��Һ����ʯ��ˮ������ˮCuSO4����Ʒ����Һ
��4������ȩ��������õ�����������ȩ��1-������Ʒ��Ϊ����1-��������С���������֪����R��CHO+NaHSO3(����) RCH(OH)SO3Na�����ڷе㣺����34�棬
RCH(OH)SO3Na�����ڷе㣺����34�棬
1-����118�棬����Ƴ������ᴿ·�ߣ�
�Լ�1Ϊ ������2Ϊ ������3Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��ͬѧչ����Ư���������ƣ�NaClO2�����о���
ʵ�����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2?3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
��1��װ�â۵������� ��
��2��װ�â��в���ClO2�Ļ�ѧ����ʽΪ ��װ�â����Ʊ�NaClO2�Ļ�ѧ����ʽΪ ��
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ����֪��ClO2��+ 4I��+4H+ =2H2O+2I2+Cl�����������û��Һ���250mL������Һ��
����ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol?L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL����֪��I2 +2S2O32��=2I��+S4O62������
��4���ﵽ�ζ��յ�ʱ������Ϊ ��
��5������Ʒ��NaClO2����������Ϊ ���ú�m��c��V�Ĵ���ʽ��ʾ����
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ǰ��һ��ʳ�������㸡�����Ϻ�����°��������������ȴ������㸡�顱�Ļ�ѧ�ɷ�ʵΪ��̼���ƣ�ʹ�ò������������ˣ���̼���Ʊ��������������岻����Σ������̼���ƣ��׳ƹ���˫��ˮ����ѧʽΪ2Na2CO3��3H2O2����һ�����Σ��ǰ�ɫ����״��ĩ�����Էֽ�Ϊ̼���ƺ������⡣ij̽��С���Ʊ���̼���Ʋ��ⶨ��Ʒ��H2O2�ĺ��������Ʊ����̺�װ��ʾ��ͼ���£�
��֪��50 ��Cʱ 2Na2CO3��3H2O2 (s) ��ʼ�ֽ�
����Ӧ 2Na2CO3 (aq) + 3H2O2 (aq) 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0
����Ӧ 2H2O2 = 2H2O + O2��
�ζ���Ӧ 6KMnO4 + 5(2Na2CO3��3H2O2) +19H2SO4 = 3K2SO4 + 6MnSO4+10Na2SO4 + 10CO2 �� + 15O2�� + 34H2O
����������Ϣ�ش��������⣺
��1���Ʋ�ͼ��֧�ܵ����ÿ����� ��
��2������ٵĹؼ��ǿ����¶ȣ����װ��ͼ�������ʩ�� ��
�� ��
��3������ҺX�м�������NaCl�������ˮ�Ҵ�����������̼���ƣ�ԭ���� ��
��4���������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ���� ��
��5���ⶨ��Ʒ��H2O2�����������ķ����ǣ�ȷ��ȡ0.2000g��̼������Ʒ��250 mL ��ƿ�У���50 mL ����ˮ�ܽ⣬�ټ�50 mL 2.000 mol��L��1 H2SO4 (H2SO4����)����0.002000mol��L��1 KMnO4����Һ�ζ����յ�ʱ����30.00 mL��
�ٵζ�ǰ���ζ�������KMnO4����Һ��ϴ2��3�Σ���ϴ�IJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4�� ��
��������Ʒ��H2O2�����������������ʽΪ ��ֻ�г���ʽ�������κ����㣡H2O2��ʽ��Ϊ34.00����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С��Ϊ��֤������ԭ��SO2��I����Fe2+�Ļ�ԭ��ǿ����˳��Ϊ��
SO2��I����Fe2+������ͼ��ʾװ�ý���ʵ�飨���Ⱥͼг�װ�����ԣ��������Ѽ��飩��
��1������ʵ����Ҫ���ȵ�װ����������������������
��2��D�У��ձ�ʢ�ŵ�����������������������������
��3��ʵ�鿪ʼʱ���ر�K1����K2��ͨ������������д��B�з�����Ӧ�����ӷ���ʽ��________________������������������������������
��Ϊ��֤I���Ļ�ԭ�Ա�Fe2+ǿ��ȡ��3����B�е�������Һ���Թ��У�Ӧ�����Թ��е�����________�������� ������Һ������������������������������������������������������������
���ڣ�3����Ϊ�˱Ƚ�SO2��I���Ļ�ԭ��ǿ�����������IJ�������������
A���ر�K2��K1��������
B���ر�K1��K2������������
C��ͬʱ��K1��K2
��4������K2��ͨ��Cl2ǡ�ý�Fe2+��I��ȫ���������ٴ�K1ͨ��SO2����Fe3+ȫ��ת��ΪFe2+ʱ����ʱͨ���SO2���������״���£�Ϊ�� ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���Ӧ�Ľ��۲���ȷ����

| A���������Zn��Cuԭ��� |
| B������֤���ǽ�����Cl>C>Si |
C����˵����Ӧ2NO2(g) N2O4(g)����H<0 N2O4(g)����H<0 |
| D���ܰ�ɫ����ΪBaSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij��ѧС��Ϊ�ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
����һ��ͭ������� �ⶨ������������
�ⶨ������������
��������ͭ������� �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ���� (����)��
| A����ҺA����ҺB�������������NaOH��Һ |
| B������ҺBѡ��Ũ���ᣬ���ͭ����������ƫ�� |
| C������һ���ܲ�������������������ʣ��ͭ |
| D��ʵ�����з����������ʵʩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com