
ŠĀŠĶøߊ§µÄ¼×ĶéČ¼ĮĻµē³Ų²ÉÓĆ²¬ĪŖµē¼«²ÄĮĻ£¬Į½µē¼«ÉĻ·Ö±šĶØČėCH4ŗĶO2 £¬µē½āÖŹĪŖKOHČÜŅŗ”£Ä³ŃŠ¾æŠ”×齫Į½øö¼×ĶéČ¼ĮĻµē³Ų“®ĮŖŗó×÷ĪŖµēŌ“£¬½ųŠŠ±„ŗĶŃõ»ÆÄĘĻ½Ņŗµē½āŹµŃ飬ČēĶ¼ĖłŹ¾”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼×ĶéČ¼ĮĻµē³ŲÕż¼«”¢øŗ¼«µÄµē¼«·“Ó¦·Ö±šĪŖ ”¢ ”£[Ą“Ō“:ѧæĘĶųZXXK]
(2)±ÕŗĻKæŖ¹Ųŗó£¬a”¢bµē¼«ÉĻ¾łÓŠĘųĢå²śÉś£®ĘäÖŠbµē¼«ÉĻµĆµ½µÄŹĒ £¬µē½āĀČ»ÆÄĘČÜŅŗµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ £»
(3)ČōĆæøöµē³Ų¼×ĶéĶØČēĮæĪŖ1 L(±ź×¼×“æö)£¬ĒŅ·“Ó¦ĶźČ«£¬ŌņĄķĀŪÉĻĶعżµē½ā³ŲµÄµēĮæĪŖ (·ØĄµŚ³£ŹżF=9.65”Įl04C ”¤ mol-1ĮŠŹ½¼ĘĖć)£¬×ī¶ąÄܲśÉśµÄĀČĘųĢå»żĪŖ L(±ź×¼×“æö)”£
 ½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĶ¼ŹĒŅ»øö»Æѧ¹ż³ĢµÄŹ¾ŅāĶ¼”£
£Ø1£©Ķ¼ÖŠŅŅ³ŲŹĒ ×°ÖĆ”£
£Ø2£©c£ØPt£©µē¼«µÄĆū³ĘŹĒ ”£
£Ø3£©Š“³öĶØČėCH3OHµÄµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ ”£
£Ø4£©ŅŅ³ŲÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©µ±ŅŅ³ŲÖŠB£ØAg£©¼«µÄÖŹĮæŌö¼Ó5£®40gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄO2 mL£Ø±ź×¼×“æöĻĀ£©£»“ĖŹ±±ū³Ųijµē¼«Īö³ö1.6gij½šŹō£¬Ōņ±ūÖŠµÄijŃĪČÜŅŗæÉÄÜŹĒ £ØĢīŠņŗÅ£©
| A£®MgSO4 | B£®CuSO4 | C£®NaCL | D£®AgNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø11·Ö)¼×”¢ŅŅĮ½³Ųµē¼«²ÄĮĻ¶¼ŹĒĢś°ōÓėĢ¼°ō£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōĮ½³ŲÖŠ¾łĪŖCuSO4ČÜŅŗ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗó£ŗ
¢ŁÓŠŗģÉ«ĪļÖŹĪö³öµÄŹĒ¼×³ŲÖŠµÄ________°ō£¬ŅŅ³ŲÖŠµÄ________°ō”£
¢ŚŅŅ³ŲÖŠŃō¼«µÄµē¼«·“Ó¦Ź½ŹĒ_______________________________________________ _”£
£Ø2£©ČōĮ½³ŲÖŠ¾łĪŖ±„ŗĶNaClČÜŅŗ£ŗ
¢ŁŠ“³öŅŅ³ŲÖŠ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½__________________________________________”£
¢Ś¼×³ŲÖŠĢ¼¼«ÉĻµē¼«·“Ó¦Ź½ŹĒ____________________£¬ŅŅ³ŲÖŠĢ¼¼«ÉĻµē¼«·“Ó¦ŹōÓŚ____________(Ģī”°Ńõ»Æ·“Ó¦”±»ņ”°»¹Ō·“Ó¦”±)”£
¢ŪČōŅŅ³Ų×ŖŅĘ0.02 mol e£ŗóĶ£Ö¹ŹµŃ飬øĆ³ŲÖŠČÜŅŗĢå»żŹĒ200 mL£¬ŌņČÜŅŗ»ģŌČŗóµÄpH£½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°“ĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ飬²¢»Ų“šĻĀĮŠĪŹĢā£ŗ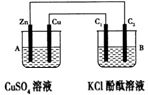
£Ø1£©ÅŠ¶Ļ×°ÖƵÄĆū³Ę£ŗA³ŲĪŖ £¬B³ŲĪŖ ”£
£Ø2£©Šæ¼«ĪŖ ¼«£¬µē¼«·“Ó¦Ź½ĪŖ £»Ķ¼«ĪŖ ¼«£¬µē¼«·“Ó¦Ź½ĪŖ £»ŹÆÄ«°ōC1ĪŖ ¼«£¬µē¼«·“Ó¦Ź½ĪŖ £»ŹÆÄ«°ōC2ø½½ü·¢ÉśµÄŹµŃéĻÖĻóĪŖ £¬·“Ó¦½įŹųŗó£¬B³ŲČÜŅŗµÄpHÖµ ”£(Ōö“󔢼õŠ””¢²»±ä£¬ŗöĀŌĘųĢåČÜÓŚĖ®) ”£
£Ø3£©µ±C2¼«Īö³ö224 mLĘųĢå£Ø±ź×¼×“æöĻĀ£©£¬ŠæµÄÖŹĮæ £ØŌö¼Ó»ņ¼õÉŁ£© g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŹµŃ銔×éµÄĶ¬Ń§²ÉÓĆČēĶ¼ĖłŹ¾×°ÖĆĄ“½ųŠŠÓŠ¹Ų»ÆѧŹµŃ飬ĒėĢī³äĻĀĮŠæÕøń”£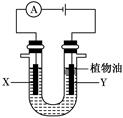
(1)ČōUŠĪ¹ÜÖŠŹ¢ÓŠĮņĖįÄĘČÜŅŗ£¬X”¢Yµē¼«·Ö±šĪŖŹÆÄ«ŗĶĢś°ō£¬µē½ā¹ż³ĢÖŠ³öĻÖµÄĻÖĻóŹĒ ”£
UŠĪ¹ÜÖŠ¼ÓČėµÄÉŁĮæÖ²ĪļÓĶ×÷ÓĆŹĒ ”£
(2)µē½āŅ»¶ĪŹ±¼äŗó£¬Ä³Ķ¬Ń§½«µēŌ“·“½Ó£¬“ĖŹ±³öĻÖµÄĻÖĻóŹĒ ”£ÓŠ¹ŲµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦2Ag£«(aq)£«Cu(s)===Cu2£«(aq)£«2Ag(s)Éč¼ĘµÄŌµē³ŲČēĻĀĶ¼ĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)µē¼«XµÄ²ÄĮĻŹĒ £¬µē½āÖŹČÜŅŗYŹĒ £»
£Ø2£©Ņųµē¼«ĪŖµē³ŲµÄ ¼«£¬·¢ÉśµÄµē¼«·“Ó¦ĪŖ £» Xµē¼«ÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ ”£
(3)ĶāµēĀ·ÖŠµÄµē×ÓŹĒ“Ó µē¼«Į÷Ļņ µē¼«”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£¬p”¢qĪŖÖ±Į÷µēŌ“µÄĮ½¼«£¬AÓɽšŹōµ„ÖŹXÖĘ³É£¬B”¢C”¢DĪŖ²¬µē¼«£¬½ÓĶصēŌ“£¬½šŹōX³Į»żÓŚB¼«£¬Ķ¬Ź±C”¢DÉĻ²śÉśĘųÅŻ£¬ŹŌ»Ų“š£ŗ
(1)pĪŖ ¼«£¬A¼«·¢ÉśĮĖ ·“Ó¦”£
(2)CĪŖ ¼«£¬æÉŹÕ¼Æµ½ £»DĪŖ ¼«£¬æÉŹÕ¼Æµ½ ”£
(3)C¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
(4)ŌŚµē½ā¹ż³ĢÖŠ£¬²āC”¢DĮ½¼«ÉĻ²śÉśĘųĢåµÄĢå»ż£¬ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
| Ź±¼ä(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Ņõ¼«²śÉśĘųĢå µÄĢå»ż(cm3) | 6 | 12 | 20 | 29 | 39 | 49 | 59 | 69 | 79 | 89 |
| Ńō¼«²śÉśĘųĢå µÄĢå»ż(cm3) | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ”×éĶ¬Ń§ÉčĻėÓĆČēĶ¼×°ÖƵē½āĮņĖį¼ŲČÜŅŗĄ“ÖĘČ”ŃõĘų”¢ĒāĘų”¢ĮņĖįŗĶĒāŃõ»Æ¼Ų”£
(1)X¼«ÓėµēŌ“µÄ (Ģī”°Õż”±»ņ”°øŗ”±)¼«ĻąĮ¬£¬ĒāĘų“Ó (Ń”Ģī”°A”±”¢”°B”±”¢”°C”±»ņ”°D”±)æŚµ¼³ö”£
(2)Ąė×Ó½»»»Ä¤Ö»ŌŹŠķŅ»ĄąĄė×ÓĶعż£¬ŌņMĪŖ (Ģī”°ŅõĄė×Ó”±»ņ”°ŃōĄė×Ó”±£¬ĻĀĶ¬)½»»»Ä¤£¬NĪŖ ½»»»Ä¤”£
(3)Čō½«ÖʵƵÄĒāĘų”¢ŃõĘųŗĶĒāŃõ»Æ¼ŲČÜŅŗ×éŗĻĪŖĒāŃõČ¼ĮĻµē³Ų(ŹÆÄ«ĪŖµē¼«)£¬Ōņµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
(4)ČōŌŚ±ź×¼×“æöĻĀ£¬ÖʵĆ11.2 LĒāĘų£¬ŌņÉś³ÉĮņĖįµÄÖŹĮæŹĒ £¬×ŖŅʵĵē×ÓŹżĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ē¦Šīµē³ŲŹĒµäŠĶµÄæɳäµēµē³Ų£¬ĖüµÄÕżøŗ¼«øń°åŹĒ¶čŠŌ²ÄĮĻ£¬µē³Ų×Ü·“Ó¦Ź½ĪŖ£ŗ
Pb£«PbO2£«4H£«£«2SO42”Ŗ 2PbSO4£«2H2O
2PbSO4£«2H2O
Ēė»Ų“šĻĀĮŠĪŹĢā(²»æ¼ĀĒĒā”¢ŃõµÄŃõ»Æ»¹Ō)£ŗ
·ÅµēŹ±£ŗÕż¼«µÄµē¼«·“Ó¦Ź½ŹĒ______________________________£»µē½āŅŗÖŠH2SO4µÄÅØ¶Č½«±ä________£»µ±ĶāµēĀ·Ķعż1 molµē×ÓŹ±£¬ĄķĀŪÉĻøŗ¼«°åµÄÖŹĮæŌö¼Ó________g”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com