
| ŠņŗÅ | ĒāŃõ»Æ±µµÄĢå»ż/mL | ĮņĖįĒāÄʵÄĢå»ż/mL | ČÜŅŗµÄpH |
| ¢Ł | 33.00 | 0.00 | 8 |
| ¢Ś | 33.00 | x | 7 |
| ¢Ū | 33.00 | 33.00 | 6 |
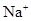 )£¾c(
)£¾c(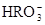 )£¾c(
)£¾c(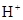 )£¾c(
)£¾c(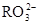 )£¾c(
)£¾c(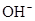 )
)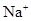 )£«c(
)£«c(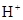 )£½c(
)£½c(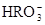 )£«2c(
)£«2c(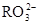 )£«c(
)£«c(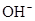 )
)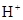 )£«c(
)£«c(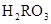 )£½c(
)£½c(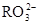 )£«c(
)£«c(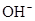 )
)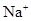 )Ӣc(
)Ӣc(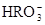 )Ӣc(
)”¢c(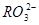 )·Ö±šĻąµČ
)·Ö±šĻąµČ


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
 HCO3-+H+µÄĘ½ŗā³£ŹżK1=______£®£ØŅŃÖŖ£ŗ10-5.60=2.5”Į10-6£©
HCO3-+H+µÄĘ½ŗā³£ŹżK1=______£®£ØŅŃÖŖ£ŗ10-5.60=2.5”Į10-6£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
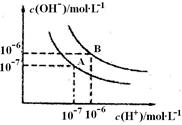
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®0.1mol/LNaHCO3ČÜŅŗÓė0.1mol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(Na£«)£¾c(CO32£)£¾c(HCO3£)£¾c(OH£) |
| B£®20ml0.1mol/LCH3COONaČÜŅŗÓė10ml0.1mol/LHClČÜŅŗ»ģŗĻŗó³ŹĖįŠŌ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(CH3COO£)£¾c(Cl£)£¾c(CH3COOH)£¾c(H£«) |
| C£®ŹŅĪĀĻĀ£¬pH£½2µÄŃĪĖįÓėpH£½12µÄ°±Ė®µČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(Cl£)£¾c(H£«)£¾c(NH4£«)£¾c(OH£) |
| D£®0.1mol/LCH3COOHČÜŅŗÓė0.1mol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 CO2(g)+H2(g) ”÷H£¼0”£ŌŚ850”ꏱ£¬Ę½ŗā³£ŹżK=1”£
CO2(g)+H2(g) ”÷H£¼0”£ŌŚ850”ꏱ£¬Ę½ŗā³£ŹżK=1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
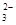 £©+c£ØHCO
£©+c£ØHCO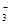 £©]
£©]| A£®¢Ł¢Ü | B£®¢Ś¢Ū | C£®¢Ł¢Ū | D£®¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CH3OH(g)£«H2O(g)£¬ŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬æ¼²ģĪĀ¶Č¶Ō·“Ó¦µÄÓ°Ļģ£¬ŹµŃé½į¹ūČēĻĀĶ¼ĖłŹ¾(×¢£ŗT1”¢T2¾ł“óÓŚ300 ”ę)£ŗ
CH3OH(g)£«H2O(g)£¬ŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬æ¼²ģĪĀ¶Č¶Ō·“Ó¦µÄÓ°Ļģ£¬ŹµŃé½į¹ūČēĻĀĶ¼ĖłŹ¾(×¢£ŗT1”¢T2¾ł“óÓŚ300 ”ę)£ŗ
 2H2O £« ”£
2H2O £« ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®c(CH3COOH)£½0.01mol”¤L-1 |
| B£®c(H+)£½c(CH3COO-) |
| C£®¼ÓĖ®Ļ”ŹĶ100±¶ŗó£¬ČÜŅŗpH£½4 |
| D£®“×ĖįµÄµēĄė³£ŹżĪŖKa£¬CH3COO-µÄĖ®½ā³£ŹżĪŖKh£¬ŌņKa”¤Kh=Kw |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com