
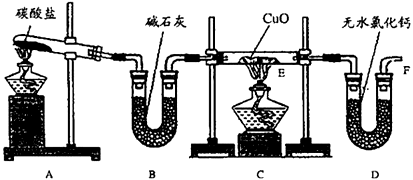
��10�֣�ʵ��������������NH3�ڼ��ȵ���������CuO��ַ�Ӧ����Cu��N2��H2O�����ⶨCu�����ԭ��������װ��ͼ���£�

��1�����Ӻ�װ�ú����װ�������Եķ�����
��
��2����װ��A��B��ȡ����������İ��������Թ���̼���εĻ�ѧʽΪ�� ��װ��B�м�ʯ�ҵ������ǣ� ��
��3��ʵ���й۲쵽Cװ�õ�E���в����������У� ��E���з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a��
��ʵ��ǰE�ܺ�CuO��������b��
�۳�ַ�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����£�������������
�ܳ�ַ�Ӧ��D�ܼ���ʢ���ʵ�������d
��ѡ������������г�����Cu�����ԭ�������ļ���ʽ����Cu�⣬�����漰����Ԫ�ص����ԭ��������Ϊ��֪����Ar(Cu)== ��
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
| 20V |
| 7 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡͬ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ʵ��������������NH3�ڼ��ȵ���������CuO��ַ�Ӧ����Cu��N2��H2O�����ⶨCu�����ԭ��������װ��ͼ���£�

��1�����Ӻ�װ�ú����װ�������Եķ�����
��
��2����װ��A��B��ȡ����������İ��������Թ���̼���εĻ�ѧʽΪ�� ��װ��B�м�ʯ�ҵ������ǣ� ��
��3��ʵ���й۲쵽Cװ�õ�E���в����������У� ��E���з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a��
��ʵ��ǰE�ܺ�CuO��������b��
�۳�ַ�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����£�������������
�ܳ�ַ�Ӧ��D�ܼ���ʢ���ʵ�������d
��ѡ������������г�����Cu�����ԭ�������ļ���ʽ����Cu�⣬�����漰����Ԫ�ص����ԭ��������Ϊ��֪����Ar(Cu)== ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com