
��ú����ȼú�糧�ķ�������Ҫ�ɷ�ΪSiO2��Al2O3��Fe2O3��C�ȡ�ʵ����ģ�ҵ�ӷ�ú����ȡ����Al2O3������������ͼ��

��֪�ս���̵IJ�����Ҫ�ǣ�NaAlO2��Ca2SiO4��NaFeO2��Na2SiO3��
��1��д���ս��������Ԫ��ת���Ļ�ѧ����ʽ ��
��2������aΪ��ȴ����ĥ��������ĥ��Ŀ���� ��
��3�����������У�NaFeO2����ȫˮ�⣬ˮ�ⷴӦ�����ӷ���ʽΪ ��
��4������b�������� �����õIJ��������� �� ���ձ���
��5����̼����ʱ���ɳ����������Ļ�ѧʽΪ ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ������ˮ��ɲ���ij�л�������A��A�ڲ�ͬ�������������£���������B(C6H12O7)��C(C6H10O8)��B��C�����ܷ���������Ӧ��A��B��C�����Ա�ǿ��ԭ����ԭΪD(C6H14O6)��B��ˮ�ɵõ���Ԫ�����������E����Ԫ�����������F����֪��������ʱ����������״�����RCHO���ף�R—CH2OH��֮��CHOHRR���ѡ�
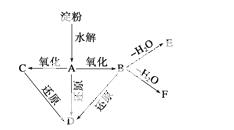
�������пո�����дA��B��C��D��E��F�Ľṹ��ʽ��
A��________________________��B��________________________________��
C��________________________��D��_______________________________��
E��________________________��F��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ӫ��Ʒ��ҩƷ���DZ�֤���ཡ������ȱ�ٵ����ʣ������ʺ��Ʒ��ǻ�ѧ�о�����Ҫ���ݡ�
��֪�Ұ�����һ�����������ȱ�ٵİ����ᣬ���Ľṹ��ʽ�ǣ�
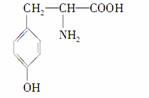
(1)�Ұ����ܷ����Ļ�ѧ��Ӧ������________��
A��ȡ����Ӧ B��������Ӧ
C��������Ӧ D���кͷ�Ӧ
(2)��֪����������Ӧ��д���Ұ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(3)д���Ұ���������Ӧ�γɶ��ĵķ���ʽ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
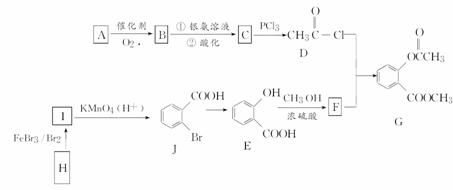
�л���G��һ��ҽҩ�м��壬��ͨ����ͼ��ʾ·�ߺϳɡ�A��ʯ�ͻ�������Ҫ��Ʒ�ҷ���������ԭ����ͬһƽ���ϣ�H�ķ���ʽ��C7H8��
��֪��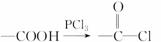
��ش��������⣺
(1)A�Ľṹ��ʽ��________��
(2)H��I�Ļ�ѧ��Ӧ����ʽΪ
________��B��������Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(3)C��D�ķ�Ӧ������________��I��J�ķ�Ӧ������________��
(4)����E������һ�������·������Ӽ���ˮ����һ�ֻ�״���Ľṹ��ʽ��________________________________________________________________________��
(5)��������������F��ͬ���칹��(��F)����________(������)�֡�
�������Ȼ�����Һ������ɫ��Ӧ
�ں�����ȷ�������������COO�ṹ
�۱�����������ȡ����
��������̼��������Һ��Ӧ�Һ˴Ź������ײⶨ��5�����շ��ͬ���칹��Ľṹ��ʽΪ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������ֵ��������������ȷ����(����)
A�����³�ѹ�£�46 g�л���C2H6O�к��м��Լ�����Ŀһ��Ϊ7NA
B����״���£�22.4 L���Ȼ�̼�������еĹ��ۼ���ĿΪ4NA
C����״���£�5.6 L NO��5.6 L O2��ɵĻ������������ԭ����ΪNA
D�����³�ѹ�£�33.6 L������56 g����ַ�Ӧ��ת�Ƶĵ�����Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͪ�������п��������ԣ�6���ǻ���ͪ������ĺϳ�·�����£�
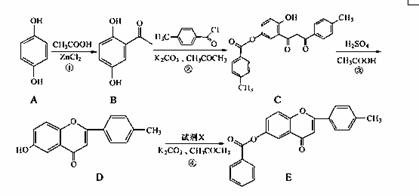
��ش��������⣺
��1��������B�еĺ���������Ϊ �� �������ƣ���
��2����Ӧ�����漰���ķ�Ӧ������ˮ�ⷴӦ�� �� ��
��3����Ӧ���м�����Լ�X�ķ���ʽΪC7H5OCl��X�Ľṹ��ʽΪ ��
��4��B��һ��ͬ���칹����������������
���ܷ���������Ӧ����ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ��
��������4�ֲ�ͬ��ѧ�������⡣
д����ͬ���칹��Ľṹ��ʽ�� ��
��5��д����Ӧ�ٵĻ�ѧ����ʽ ��
��6����֪�� ��
��
��������֪ʶ����������Ϣ��д����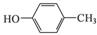 ��CH3COOHΪԭ���Ʊ�
��CH3COOHΪԭ���Ʊ�
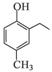 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
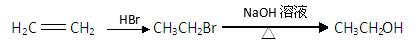
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ֵ��������ʾ���͵�������������������ı���̶���С��һ�㽫������ֵ�궨Ϊ100����ͼ����������ӵ����ģ�ͣ����������ϵͳ����Ϊ(����)
A��1,1,3,3�ļ����� B��2,2,4��������
C��2,4,4�������� D��2,3,4��������
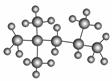
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100mL��FeBr2��Һ�У�ͨ���״����Cl2 5.04L��Cl2ȫ������ԭ�������Һ��c(Br-)=c(Cl-)����ԭFeBr2��Һ�����ʵ���Ũ����
A��0.75mol/L B��1.5mol/L C��2mol/L D��3mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CuSO4��Һ����μ���KI��Һ���������۲쵽������ɫ����CuI����ɫ��Һ��Ϊ��ɫ������Ӧ�����Һ��ͨ�������SO2���壬��Һ�����ɫ��������˵����ȷ���ǣ� ��
A��ͨ��22.4 L SO2�μӷ�Ӧʱ����2 NA�����ӷ���ת��
B��ͨ��SO2����Һ�����ɫ��������SO2��Ư����
C���μ�KI��Һʱ��KI��������CuI����������
D������ʵ�������£����ʵ������ԣ�Cu2+��I2��SO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com