
����Ŀ����֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣
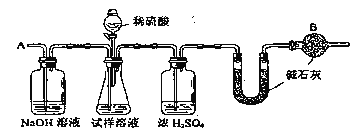
��Ҫʵ�鲽�����£��ٰ�ͼ��װ������������װ�õ������ԣ�
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�bg��
�ܴӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ��
�ݴӵ���A����������һ�����Ŀ�����
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g��
���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g��
��ش��������⣺
��1����������ƽ����ҩƷʱ�������ƽ��ָ������ƫת��˵��______��
��2��ͼ��װ���и����B��������_____��
��3���������Һ©���е����ỻ��Ũ����ͬ���������ᣬ���ԵĽ��____����ƫ�ߡ�ƫ�ͻ䣩��
��4������ݵ�Ŀ����________��
��5�������д�������������ļ���ʽΪ______��
���𰸡�ҩƷ�����������˶��� ��ֹ�����е�ˮ�ֻ������̼����U���� ƫ�� ��װ���еĶ�����̼ȫ������U���У���С��� 106��d-b��/44a
��������
��1��������ƽ����ʱ�������������ԭ���ǣ�
��2�����ڿ�����Ҳ�ж�����̼��ˮ�֣������B�����þ��Dz������ǽ���ģ�
��3����������Ļӷ��Կ��ǣ�
��4�����ڷ�Ӧ������ƿ�д����ж�����̼������һ�����Ŀ�������Ϊ���������ǵģ�
��5������U�ܵ��������������������ɵĶ�����̼�����������ݶ�����̼���������̼���Ƶ�����������̼���Ƶ�����������Ʒ�������ɡ�
��1�����ڳ���ʱ�������룬����ƫ˵����Ʒ�أ������ᣬ�ʴ�Ϊ��ҩƷ�����������˶�����
��2��U���еļ�ʯ����Ϊ�����շ�Ӧ���ɵĶ�����̼����������Ҳ���ڶ�����̼�������B�����þ��Ƿ�ֹ�����еĶ�����̼��ˮ�ֽ���U�ܣ��Խ���������ʴ�Ϊ����ֹ�����е�ˮ�ֻ������̼����U���С�
��3������������лӷ��ԣ�Ҳ�����Ŷ�����̼����U�ܣ�������Ϊ�Ƕ�����̼�����Զ�����̼������ƫ�������̼���Ƶ�����Ҳ��ƫ��ģ����Խ����ƫ�ʴ�Ϊ��ƫ��
��4�����ڷ�Ӧ������ƿ�д����ж�����̼������һ�����Ŀ������ǽ������Ķ�����̼��ȫ����U�ܣ���С���ʴ�Ϊ����װ���еĶ�����̼ȫ������U���У���С��
��5������Ҫ̼���Ƶ�����ΪX����
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
X d-b
�б���ʽ��![]()
![]()
���X=![]() �����������д�������������ļ���ʽΪ
�����������д�������������ļ���ʽΪ![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��![]() ��
��


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.���º����£���2mol A�����2mol B����ͨ�����Ϊ2L���ܱ������з������·�Ӧ��2A(g)+B(g)![]() xC(g)+2D(s)��2 minʱ��Ӧ�ﵽƽ��״̬����ʱʣ��1.2mol B�������C��Ũ��Ϊ1.2mol/L��
xC(g)+2D(s)��2 minʱ��Ӧ�ﵽƽ��״̬����ʱʣ��1.2mol B�������C��Ũ��Ϊ1.2mol/L��
(1)�ӿ�ʼ��Ӧ���ﵽƽ��״̬������C��ƽ����Ӧ����Ϊ_____ mol/(L��s)��
(2)x��____��
(3)���и������Ϊ�÷�Ӧ�ﵽƽ��״̬�ı�־����____��
A.ѹǿ���ٱ仯 B.v(A����=2v(B����
C �����ܶȲ��ٱ仯 D.A�İٷֺ������ֲ���
E.A������������C����������֮��Ϊ2��1
��.ij���ײ��� Al-Ag2O ������أ����ܽ���KOH ��������ˮΪ���Һ������ܷ�ӦΪ��2Al+3Ag2O+2KOH=6Ag+2KAlO2+H2O���Իش��������⣺
(1)Ag2O Ϊ��ص�____������������������������������ӦʽΪ______��
(2)���� 1mol�����������·ʱ��������������______g��
(3)��Һ�е� OH-��________��Ǩ�ƣ�����Ag2O������Al������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���һ���֣���ش��й����⣺
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
��2���� | �� | �� | ||||||
��3���� | �� | �� | �� | �� | �� | �� |
��1��Ԫ�آ�ԭ�ӽṹʾ��ͼ___________��д���ݵ��������һ����;__________��
��2�����Тݺ͢���̬�⻯����ȶ���˳��Ϊ ______ >______(���⻯��Ļ�ѧʽ����
��3���������γ��������������Ԫ����_________����Ԫ�ط��ţ�����Ԫ�صĵ�����۵���������ˮ��Һ��Ӧ�����ӷ�Ӧ����ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(H2NNH2)��һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯����ͼ��ʾ����֪����1 mol��ѧ�����������(kJ)��N��NΪ942��O=OΪ500��N��NΪ154�������1 mol N��H�����������(kJ)�ǣ�
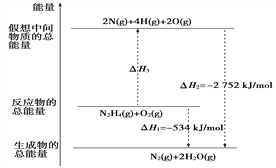
A. 194 B. 391 C. 516 D. 658
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��aN(g) ![]() bM(g)��M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
bM(g)��M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
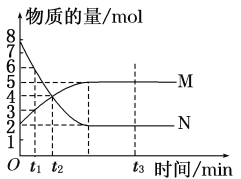
��1���˷�Ӧ�Ļ�ѧ����ʽ��a��b��________��
��2��t1��t2ʱ�̣���M��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��
��3��������������˵��������Ӧ�ﵽƽ��״̬����________________________________________________________________________��
A.��Ӧ��M��N�����ʵ���֮��Ϊ1��1
B.������������������ʱ��ı仯���仯
C.�������������ʵ�������ʱ��ı仯���仯
D.��λʱ��������a mol N��ͬʱ����b mol M
E.��������ѹǿ����ʱ��ı仯���仯
F.N��ת���ʴﵽ����ұ��ֲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaCl��Һ����һ�����������������ϣ�һ��ʱ�����Һ�θ��ǵ�Բ��������(a)�ѱ���ʴ���䰵����Һ�������γ���ɫ���(b)����ͼ��ʾ�����¸��������Ҫԭ����Һ��֮�����������ȱ�Ե�١�����˵����ȷ������ ��
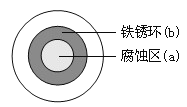
A. Һ���е�Cl�D��a����b��Ǩ��
B. Һ�α�Ե���������������ĵ缫��ӦΪ��O2��2H2O��4e��=4OH��
C. Һ���µ�Fe������ԭ��Ӧ������ʴ�����ɵ�Fe2����a����b��Ǩ�ƣ���b����OH�D�γ�Fe(OH)2����һ����������ˮ�γ�����
D. ������Ƕ��һͭ��˿�������壬��ͭ���Ӵ����μ�NaCl��Һ���������ĵ缫��ӦΪ��Cu��2e��=Cu2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����Ȼ�隣������̿��Ʊ��ߴ�̼���̡���֪�����̿����Ҫ�ɷ���MnCO3�����к�Fe��Ca��Mg��Al��Ԫ�ء����պ�IJ�����Ũ����������ٶԽ���Һ�������ӣ��õ��ľ���Һ����̼�������Һ���ɳ�����ϴ�Ӹ���ɵõ���Ʒ���ش�
��1�����չ����в����������壬һ�ֿ�ʹʪ�����ɫʯ����ֽ��죬��һ�ֿ�ʹʪ��ĺ�ɫʯ����ֽ��������д�����չ�������Ҫ��Ӧ�ķ���ʽ______________��
��2���Խ���Һ��������ʱ�����ȼ���MnO2��Fe2+ת��ΪFe3+��д���÷�Ӧ�����ӷ���ʽ______________________������Fe3+�����Լ���������__________��
��3������Һ����̼�������Һʱ��Ӧ�����ӷ���ʽΪ_____________________��
��4���������������п�ѭ��ʹ�õ�������________��
A��MnCO3 B. HCl C. NH4Cl D. ̼�����
��5���õζ����ⶨ����Һ��Mn2+�ĺ���ʱ���������м����Թ�������������ᣬ���Ȼ�����NO2���������Թ���������刺��Խ���ת��������Ⱦ�����ʶ���ȥ���÷�Ӧ�����ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У���ȫ�����ӻ������һ���ǣ� ��
A. �Ȼ�李�̼�����ơ������� B. ����ء���HNO3
C. ����þ��ʳ�Ρ�CaCO3 D. K2MnO4����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���к���ͬ�������ͬpHֵ��H2SO4��HCl��CH3COOH����ϡ��Һ��������ͬŨ�ȵ�NaOH��Һ�����ΪV1��V2��V3���� ��
A.V1��V2��V3B.V3��V2��V1
C.V3��V1��V2D.V1��V2��V3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com