
 �Ӻ�ˮ����ȡ����������ȡþ�Ĺ������漰���ļ������ʳ����µ��ܶȻ���������������ѧ��֪ʶ�ش�����ļ������⣺?
�Ӻ�ˮ����ȡ����������ȡþ�Ĺ������漰���ļ������ʳ����µ��ܶȻ���������������ѧ��֪ʶ�ش�����ļ������⣺?| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 |
| �ܶȻ� | 2.8��10-9 | 6.8��10-6 | 5.5��10-6 | 1.8��10-11 |
 CaO��CO2��?��2�֣�����д�������۷֣�
CaO��CO2��?��2�֣�����д�������۷֣� CaO��CO2����
CaO��CO2����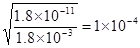 ��������ҺpH���ӦΪ10��
��������ҺpH���ӦΪ10��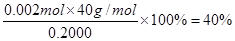 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ױ���ˮ | B���ױ��ͱ� | C����������ˮ | D������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ó���ʯ��ˮ����CO2��SO2 |
| B����KSCN��Һ����FeCl2�Ƿ���� |
| C����ʪ�����ɫʯ����ֽ��ֽ����Cl2��HCl |
| D���ù۲���ɫ�ķ�������NO��NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����̪��Һ | B���Ȼ�����Һ | C��ʯ����Һ | D��̼������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥN2�е�����O2��ͨ�����ȵ�CuO��ĩ���ռ����� |
| B����ȥCO2�е�����HCl��ͨ��Na2CO3��Һ���ռ����� |
| C����ȥFeCl2��Һ�е�����FeCl3������������м����ַ�Ӧ���� |
| D����ȥKCl��Һ�е�����MgCl2����������NaOH��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ��Ŀ�� |
| A�� | �������Һ�м�������ʯ��ʯ���� | ��֤������̼�������ǿ�� |
| B�� | �ڵ�����Һ�м���20%��ϡH2SO4ˮ�����������������Һ��������Ӧʵ�� | ��֤����ˮ���Ƿ������������� |
| C�� | ��CuSO4��Һ�м������NaOH��Һ����������ijͬѧ��Һ������ | ��֤��ͬѧ�Ƿ������� |
| D�� | ���Ҵ���Һ�в���һ�����ȱ�ڵ�ͭ˿ | ��֤�Ҵ��Ƿ�������ȩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
|
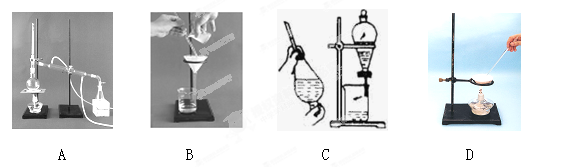 ���ݻ���������ᴿ��ԭ�����ش�������ʵ������Ҫʹ������װ�ã���A��B��C��D�����ʵ��Ŀո��У���
���ݻ���������ᴿ��ԭ�����ش�������ʵ������Ҫʹ������װ�ã���A��B��C��D�����ʵ��Ŀո��У����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com