
��12�֣� (���ʽṹ������)���ͽ��ܲ��ϸ��³����������ͻ������1987����µ��Ʊ�ͭ�����ϵ��о���ʼ�ġ����Ʊ��Ʊ�ͭ�����³������ͬʱ��żȻ�õ��˸���Ʒ������ɫ�Ĺ���ͭ�������ɵ��ǣ��������Ƿ������к��������ϵı���Ϊ�����ϡ������ϣ�����������ٸ�ʻ档 �Ʊ�ͭ���ľ����ṹ��ͼ���о����֣��˸��³������е�ͭԪ�������ּ�̬����2�ۺͣ�3�ۡ�
�Ʊ�ͭ���ľ����ṹ��ͼ���о����֣��˸��³������е�ͭԪ�������ּ�̬����2�ۺͣ�3�ۡ�
��1������ͭ�����ڱ��е�λ�ã����ں��壩
��2��д����̬Cuԭ�ӵĺ�������Ų���
��3������ͼʾ�����ṹ�����㾧����Y��Cu��Ba��Oԭ�Ӹ����ȣ�ȷ���仯ѧʽΪ��
��4����ij+1��ͭ��������Ϊ[Cu(CN)4]3-���������廥Ϊ�ȵ������һ������
��д��ѧʽ����
������+1��ͭ�Ļ��������Һ������CO��ϩ������C2H4��CH3-CH=CH2��������CH3-CH=CH2������Cԭ�ӵ��ӻ���ʽ�� �� ��
��5��������ͭ��Һ����μ��백ˮ���������۲쵽�ȳ�����ɫ���������ճ����ܽ�õ�����ɫ����Һ��д����ɫ�����ܽ�����ӷ���ʽ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����һģ��[��ѧ--ѡ��3���ʽṹ������]
��2013?����һģ��[��ѧ--ѡ��3���ʽṹ������]| 417��1030 |
| a3NA |
| 417��1030 |
| a3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | A | B | C |
| �ṹ��Ϣ | ��̬ԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��� | ��̬ԭ�ӵ�M����1�ԳɶԵ�p���� | ��̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼ� |
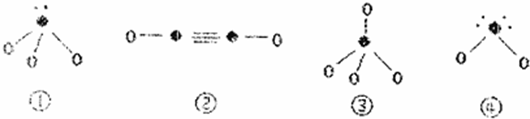
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 [��ѧ-ѡ�����ʽṹ������]����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ����������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ͬ�ĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬��
[��ѧ-ѡ�����ʽṹ������]����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ����������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ͬ�ĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
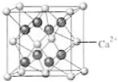 ��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ����
��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com