
���� ��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���������Ų�ʽΪ1s22s22p3��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ����BΪClԪ�ء�CΪKԪ�أ�
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ����������Ϊ2+8+18+1=29����EΪCuԪ�أ�
��5��ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ�أ���Χ�����Ų�ʽΪ1s1��2s22p2��2s22p4��3s23p3��3d64s2��
��� �⣺��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���������Ų�ʽΪ1s22s22p3��ΪNԪ�أ��ʴ�Ϊ��N��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ����BΪClԪ�ء�CΪKԪ�أ�ClԪ��ԭ�Ӻ�����Ų�ʽΪ��1s22s22p63s23p5���ʴ�Ϊ��1s22s22p63s23p5��K��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��ΪFeԪ�أ��ʴ�Ϊ��Fe��1s22s22p63s23p63d64s2��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ����������Ϊ2+8+18+1=29����EΪCuԪ�أ���������Ų�ʽΪ��1s22s22p63s23p63d104s1���ʴ�Ϊ��Cu��1s22s22p63s23p63d104s1��
��5��ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ�أ���Χ�����Ų�ʽΪ1s1��2s22p2��2s22p4��3s23p3��3d64s2������5�֣��ʴ�Ϊ��5��
���� ���⿼���������Ų����ɡ��ṹ��λ�ù�ϵ��ע�������������ԭ��������ԭ�������ع����������Ƚϻ�����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
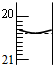 ��0.1000mol/L NaOH��Һ�ζ�����H2SO4��Һ��
��0.1000mol/L NaOH��Һ�ζ�����H2SO4��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 +CO2��g��?
+CO2��g��? +CO��g��+H2O��g����H
+CO��g��+H2O��g����H ?
? +H2��g����H1=+117.6kJ/mol
+H2��g����H1=+117.6kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
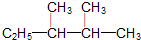 ����ϵͳ��������������2��3-�������飻
����ϵͳ��������������2��3-�������飻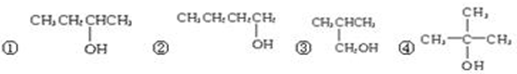
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���»��� | B�� | ���ۼ� | C�� | ��� | D�� | ���Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com