
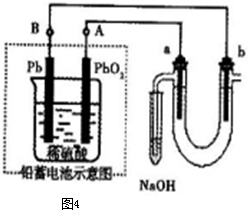
ÓŠµē½ā×°ÖĆČēĶ¼4-37”£Ķ¼ÖŠB×°ÖĆŹ¢1 L 2 mol”¤L-1 Na2SO4ČÜŅŗ£¬A×°ÖĆÖŠŹ¢?1 L 2 mol”¤L-1 AgNO3ČÜŅŗ”£Ķصēŗó£¬ČóŹŖKIµķ·ŪŹŌÖ½µÄC¶Ė±äĄ¶É«£¬µē½āŅ»¶ĪŹ±¼äŗó£¬ŹŌ»Ų“š£ŗ
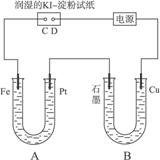
Ķ¼4-37
(1)AÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________________”£
(2)ŌŚBÖŠ¹Ū²ģµ½µÄĻÖĻóŹĒ_________________________”£
(3)ŹŅĪĀĻĀ£¬Čō“Óµē½āæŖŹ¼µ½Ź±¼äĪŖtŹ±£¬A”¢B×°ÖĆÖŠ¹²ŹÕ¼Æµ½ĘųĢå0.168 L(±ź×¼×“æö)£¬Čōµē½ā¹ż³ĢÖŠĪŽĘäĖūø±·“Ó¦·¢Éś£¬ĒŅČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę£¬ŌņŌŚtŹ±£¬AČÜŅŗµÄpHĪŖ_______”£
(1)4AgNO3+2H2O![]() 4Ag+O2ӟ+4HNO3
4Ag+O2ӟ+4HNO3
(2)ŹÆÄ«µē¼«±ķĆęÓŠĘųÅŻ²śÉś£¬Ķµē¼«ÖÜĪ§ČÜŅŗ±äĄ¶É«£¬Ņ»¶ĪŹ±¼äŗóUŠĪ¹ÜĻĀ²æÓŠĄ¶É«³Įµķ²śÉś (3)2
C¶Ė±äĄ¶ĖµĆ÷·¢ÉśĮĖ·“Ó¦2I--2e-====I2£¬Ź¹µķ·Ū±äĄ¶£¬¹ŹC¶ĖĪŖŃō¼«£¬D¶ĖĪŖŅõ¼«£¬¾Ż“ĖŅąæÉČ·¶ØµēŌ“µÄÕż”¢øŗ¼«£¬¼°A”¢BµÄŅõ”¢Ńō¼«”£¶ŌÓŚB£¬ŹÆÄ«ĪŖŅõ¼«£¬·¢Éś2H++2e-====H2”ü²¢²śÉśOH-£¬¶ųCuĪŖŃō¼«£¬¹Ź²»¶ĻČܽā”£¶ŌÓŚA£¬FeĪŖŅõ¼«£¬ÓŠAgĪö³ö£¬PtĪŖŃō¼«ÓŠO2²śÉś”£ÓÉÓŚ²śÉśµÄO2ĪŖH2µÄ![]() £¬¹ŹO2µÄĪļÖŹµÄĮæĪŖ
£¬¹ŹO2µÄĪļÖŹµÄĮæĪŖ![]() =0.0025 mol£¬Ķ¬Ź±»įŹ¹AÖŠH+±äĪŖ4”Į0.0025 mol=0.01 mol£¬ĖłŅŌAÖŠc(H+)=0.01 mol”¤L-1£¬pH=2”£
=0.0025 mol£¬Ķ¬Ź±»įŹ¹AÖŠH+±äĪŖ4”Į0.0025 mol=0.01 mol£¬ĖłŅŌAÖŠc(H+)=0.01 mol”¤L-1£¬pH=2”£


 ĘŚÄ©±¦µäµ„ŌŖ¼ģ²ā·ÖĄąø“Ļ°¾ķĻµĮŠ“š°ø
ĘŚÄ©±¦µäµ„ŌŖ¼ģ²ā·ÖĄąø“Ļ°¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ”÷ |
| ||
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗžÄĻŹ”ÓĄÖŻ°ĖÖŠ2007½ģøßČż»ÆѧµŚČż“ĪŌĀæ¼ŹŌ¾ķ ĢāŠĶ£ŗ022
| |||||||||||||||||||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķ¼4-37
(1)AÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________________”£
(2)ŌŚBÖŠ¹Ū²ģµ½µÄĻÖĻóŹĒ_________________________”£
(3)ŹŅĪĀĻĀ£¬Čō“Óµē½āæŖŹ¼µ½Ź±¼äĪŖtŹ±£¬A”¢B×°ÖĆÖŠ¹²ŹÕ¼Æµ½ĘųĢå0.168 L(±ź×¼×“æö)£¬Čōµē½ā¹ż³ĢÖŠĪŽĘäĖūø±·“Ó¦·¢Éś£¬ĒŅČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę£¬ŌņŌŚtŹ±£¬AČÜŅŗµÄpHĪŖ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓ±±Ź”ĢĘɽŹŠøßȿğ¼¶Ćžµ×æ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
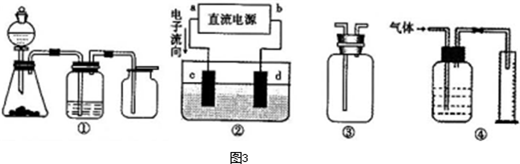
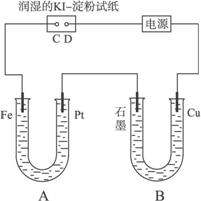
ŠĀŠĶøߊ§µÄ¼×ĶéČ¼ĮĻµē³Ų²ÉÓĆ²¬ĪŖµē¼«²ÄĮĻ£¬Į½µē¼«ÉĻ·Ö±šĶØČėCH4ŗĶO2£¬µē½āÖŹĪŖKOHČÜŅŗ”£Ä³ŃŠ¾æŠ”×齫¼×ĶéČ¼ĮĻµē³Ų×÷ĪŖµēŌ“½ųŠŠĀČ»ÆĆ¾ČÜŅŗ¹ź½āŹµŃ飬µē½ā×°ÖĆČēĶ¼ĖłŹ¾”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×ĶéČ¼ĮĻµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ ”£
£Ø2£©±ÕŗĻæŖ¹ŲKŗó£¬a”¢bµē¼«ÉĻ¾łÓŠĘųĢå²śÉś£¬ĘäÖŠaµē¼«ÉĻµÄĘųĢåæÉÓĆ ¼ģŃ飬bµē¼«ÉĻµĆµ½µÄĘųĢåŹĒ £¬µē½āĀČ»ÆĆ¾ČÜŅŗµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©Čō¼×ĶéĶØČėĮæĪŖ1.12 L£Ø±ź×¼×“æö£©£¬ĒŅ·“Ó¦ĶźČ«£¬ŌņĄķĀŪÉĻĶعżµē½ā³ŲµÄµē×ÓµÄĪļÖŹµÄĮæĪŖ £¬²śÉśµÄĀČĘųĢå»żĪŖ L£Ø±ź×¼×“æö£©”£
£Ø4£©ŅŃÖŖ³£ĪĀ³£Ń¹ĻĀ£¬0.25molCH4ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶH2OŹ±£¬·Å³ö222.5kJČČĮ棬ĒėŠ“³öCH4Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
ŅŃÖŖ£»¢ŁC£ØŹÆÄ«£©+O2£Øg£©=CO2£Øg£©”÷H1=-393£®5kJ/mol
¢Ś2H2£Øg£©+O2£Øg£©=2H2O£Øl£©”÷H2=-571£®6kJ/mol
¼ĘĖć£ŗC£ØŹÆÄ«£©ÓėH2£Øg£©·“Ӧɜ³É1molCH4£Øg£©µÄ”÷H= ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com