

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �� | �� ����Ԫ��״����� | �� �������۷�Ӧ���ɣ� | �� �����Ӿ۷�Ӧ���ɣ� |
| | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
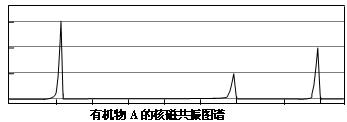
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
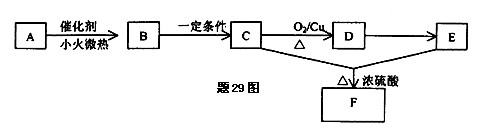
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
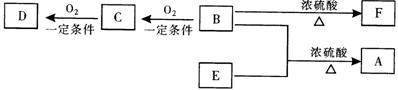
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
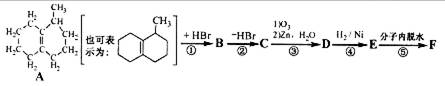
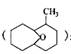 �⣬���õ�����ͬ���칹�壬
�⣬���õ�����ͬ���칹�壬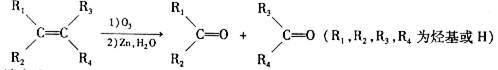
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 �����������NaOH��Һ
�����������NaOH��Һ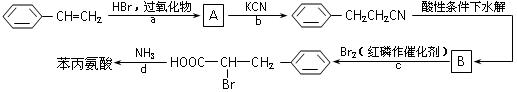

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
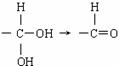 �����
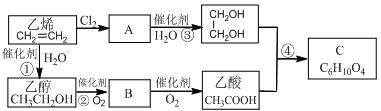
���л���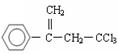 ��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ졣�����йظ��л����˵������ȷ���ǣ� ��
��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ졣�����йظ��л����˵������ȷ���ǣ� ��| A������±��������ʹ���Ը��������Һ��������Ȼ�̼��Һ��ɫ |
| B�������ʼ��������칹��Ҳ��˳���칹 |
| C���ڼ��������³��ˮ�⣬������������ |
| D��1mol ��������һ�������¿���4molH2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com