
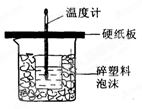
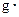 �棩�������NaOH��Һ���ܶ���Ϊ����1
�棩�������NaOH��Һ���ܶ���Ϊ����1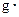
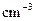 ��
�� �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ָ���³�ѹ�£�2������Ӻ�1�������ӷ�Ӧ����2��ˮ���ӣ��ų�����571.6 kJ |
| B����ָ���³�ѹ�£�2 mol H2(g)��1 mol O2(g)��Ӧ����2 mol H2O(l)���ų�����571.6 kJ |
| C����ָ��״���£�2 mol H2O(l)�ֽ�Ϊ2 mol H2(g)��1 mol O2(g)����������571.6 kJ |
| D����ָ���³�ѹ�£�2 mol H2(g)��1 mol O2(g)��Ӧ����2 mol H2O(g)���ų�����571.6 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q1=Q2 | B��Q1��Q2 | C��Q1��Q2 | D�����Ƚ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
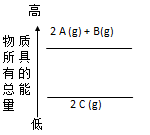
| A��2A��g��+ B(g)=2C��g����H=a��a��0�� |
| B��2A��g��+ B(g)=2C��g����H=a��a��0�� |
| C��2A + B =" 2" C ��H=a��a��0�� |
| D��2C ="2A" + B��H=a��a��0�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
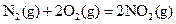 ��H=+67.7k
��H=+67.7k J��mol-1
J��mol-1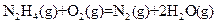 ��H=-543kJ��mol-1
��H=-543kJ��mol-1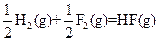 ��H=-269kJ��mol-1
��H=-269kJ��mol-1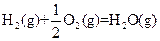 ��H=-242kJ��mol-1
��H=-242kJ��mol-1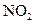 ��Ӧ���Ȼ�ѧ����ʽΪ�� ��
��Ӧ���Ȼ�ѧ����ʽΪ�� ��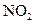 ������������Ӧ�ͷŵ����������ºͷ�����Ӧ���Ȼ�ѧ����ʽΪ�� ��
������������Ӧ�ͷŵ����������ºͷ�����Ӧ���Ȼ�ѧ����ʽΪ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C8H18��1����22.5O2��g����8CO2��g����9H2O��g����H����48.40kJ��mol��1 |
| B��C8H18��1����22.5O2��g����8CO2��g����9H2O��1����H����5518kJ��mol��1 |
| C��C8H18��1����22.5O2��g����8CO2��g����9H2O��1����H����5518kJ��mol��1 |
| D��C8H18��1����22.5O2��g����8CO2��g����9H2O��1����H����48.40kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(l)DH=+28.7kJ/mol |
| B��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(1)DH=��28.7kJ/mol |
| C��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(1)DH=+57.4kJ/mol |
| D��NaOH(aq)+HCl(aq) = NaCl(aq)��H2O(1)DH=��57.4kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2SO3����H=��196.6kJ/mol
2SO3����H=��196.6kJ/mol ��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol"
��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol" �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��2SO2+ O2 2SO3����H=��196.6kJ/mol 2SO3����H=��196.6kJ/mol |
| B��N2(g) + 2O2(g) ="=" 2NO2(g)����H= +67.7kJ/mol |
| C��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol" |
| D��H2O(l) ="=" H2(g)��+ 1/2O2(g)������H= +285.8kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com