
K�ֱ�����йط�Ӧ��һ�ַ�Ӧ������������A��C��F��K�ǹ��壬��A��һ�ֲ�������Ԫ�ص��Σ����ȷֽ��ܵõ��������ʵ�����ȵIJ�� A���Ⱥ����ɵ�����������ͨ����ʯ�ң�ֻʣ������B��BΪ��ɫ�д̼�����ζ�����壩����ͨ��Ũ������ֻʣ������D���ش������⣺
��1��д��C�ĵ���ʽ ��
��2��д������A����Һ������NaOH��Һ��Ӧ�����ӷ���ʽ
��
��3��д��ʵ������ȡB�Ļ�ѧ����ʽ
��
��4��д��N��K��Ӧ����ʽ
��
��5������̽��ڻ��Ǵ����м�������M��������ʾ��M��������ԭ�ӷ��ӣ�����Է�������Ϊ60���ڵ�����M�ֽ⡣��ĩ״��KSCN��Ũ������һ�������¿ɵõ�����M�������������Σ�����������ʵ���֮����1��1��1��������M�Ľṹʽ�� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
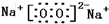
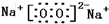
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
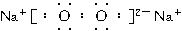
��ش��������⣺
(1)д��C�ĵ���ʽ��_______________��
(2)д��ʵ������ȡB�Ļ�ѧ����ʽ��_____________________________________________��
(3)д��N��K��Ӧ�����ӷ�Ӧ����ʽ��___________________________________________��
(4)���������ͨ����ʯ�ҵõ�������B��ͨ��Ũ����õ�������D������֮����7��11�������A��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
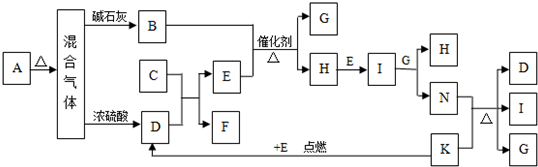
��ͼ�е�B��K�ֱ�����йط�Ӧ��һ�ַ�Ӧ������������A��C��F��K�ǹ��壻E�dz��������嵥�ʣ�I�Ǻ���ɫ����̬�������̬����A���������Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B����ͨ��Ũ������ֻʣ������D�������ʼ��ת����ϵ����ͼ��ʾ��
��ش��������⣺
(1)B�Ļ�ѧʽΪ_____________________��D�ĵ���ʽ_________________________.
(2) д��ʵ���Ҽ���A�����к��е������ӵķ��� ��
(3) д��ʵ������ȡB�Ļ�ѧ����ʽ ��
(4) ��0.01mol Dͨ�� 1L 0.01mol/L F��Һ�У�������Һ����������Ũ���ɴ�С����˳��Ϊ ��
(5) д��N��ϡ��Һ����������۷�Ӧ�����ӷ���ʽ ��
(6) ���������ͨ����ʯ�ҵõ�������B��ͨ��Ũ����õ�������D�����ʵ���֮����8��5���������ʵ����Ĺ�ϵ��ʾ�˹���A�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����������¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
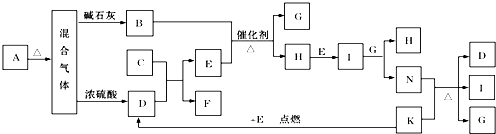
��ͼ�е�B��K�ֱ�����йط�Ӧ��һ�ַ�Ӧ������������A��C��F��K�ǹ��壻E�dz��������嵥�ʶ�I�Ǻ���ɫ����̬�������̬����A���Ⱥ����ɵ�����������ͨ����ʯ��ֻʣ������B����ͨ��Ũ������ֻʣ������D�������ʼ��ת����ϵ����ͼ��ʾ��

��ش��������⣺
(1) д��ʵ���Ҽ���A�����к��е������ӵķ��� ��
(2) B��E��Ӧ�õ�1molH�����ʱת�Ƶ��ӵ����ʵ���Ϊ mol��
(3) д��ʵ������ȡB�Ļ�ѧ����ʽ ��
(4) д��N��ϡ��Һ����������۷�Ӧ�����ӷ���ʽ ��
(5) ���������ͨ����ʯ�ҵõ�������B��ͨ��Ũ����õ�������D�����ʵ���֮����8��5���������ʵ����Ĺ�ϵ��ʾ�˹���A�����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com