
 ��
��
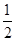 O2(g)=CO2(g)��2H2(g)��H����192��9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)��H����192��9 kJ��mol��1| ��ѧ�� | P��P | P��O | O=O | P=O |
| ����/kJ��mol��1 | a | b | c | x |
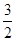 O2(g)=CO2(g)��2H2O(l)����H����764��7 kJ��mol��1
O2(g)=CO2(g)��2H2O(l)����H����764��7 kJ��mol��1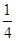 (d��6a��5c��12b)
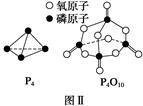
(d��6a��5c��12b) P4O10�����ͼ���а�������ȫȼ�ղ���Ľṹ�����ݡ���Ӧ�ȣ���Ӧ������ܺͣ�����������ܺ͡���ȼ���ȸ���ɵõ�ʽ��6a��5c��(4x��12b)����d���ݴ˿ɵ�x��
P4O10�����ͼ���а�������ȫȼ�ղ���Ľṹ�����ݡ���Ӧ�ȣ���Ӧ������ܺͣ�����������ܺ͡���ȼ���ȸ���ɵõ�ʽ��6a��5c��(4x��12b)����d���ݴ˿ɵ�x��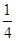 (d��6a��5c��12b)��
(d��6a��5c��12b)��

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ԭ����ͬһƽ�� |
| B��������ÿ�����ļ���Ӧ��� |
| C����ABn��Aԭ��û�й¶Ե��� |
| D��A�����ԭ������С��B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ӻ������в����ܺ��й��ۼ� |
| B���ڹ��ۻ�������Ҳ���ܺ������Ӽ� |
| C�����������Ӽ��Ļ�����һ�������ӻ����� |
| D���ɲ�ͬ�ַǽ���Ԫ����ɵĻ�������ֻ���м��Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Be2���е����Ӻ͵��� |
B�� �е����Ӻ����� �е����Ӻ����� |
| C��NaHCO3�е������Ӻ������� |
| D��Na2O2�е������Ӻ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2(g)��ȼ����Ϊ -571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C�� H2SO4(aq)�� H2SO4(aq)�� Ba(OH)2(aq)�� Ba(OH)2(aq)��  BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ���з�Ӧ�������е����������������������е������� |
| B��2N2 H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=��1000��3 kJ/mol |
| C�������缫��KOH��Һ���������Һ���ɷ�Ӧ����Ƶ�ȼ�ϵ�أ��为����ӦʽΪN2H4��4e��+4OH��=N2+4H2O |
| D�������缫��KOH��Һ���������Һ���ɷ�Ӧ����Ƶ�ȼ�ϵ�أ�����һ��ʱ���KOH��Һ��pH������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A | B | C | D |
 |  |  |  |
| ��̫���ܵ�� | ����ӵ�� | ̫���ܼ����� | ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)=H2O(l)
O2(g)=H2O(l)| A�������ĸ���Ӧ�������ȷ�Ӧ |
| B��1 molҺ̬H2����������1 mol��̬H2������ |
| C��H2��ȼ���Ȧ�HΪ��285.8 kJ��mol��1 |
D�������Һ��ȼ�յ��Ȼ�ѧ����ʽΪH2(l)�� O2(l)=H2O(g)����H����285.8 kJ��mol��1 O2(l)=H2O(g)����H����285.8 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢܢߢࡡ | B���٢ۢܢߢ� | C���٢ڢݢޢ� | D���٢ܢߢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com