
| ʵ����� | �� | �� | �� |
| �Ͻ�������mg�� | 255 | 385 | 459 |
| �������������mL�� | 280 | 336 | 336 |
���� ��1������Ũ�����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ�����ݼ��н�����������������ȹ�ϵ��������336mL������Ҫ������������ȷ����������������Ƿ�ǡ�÷�Ӧ��
������ȫ��Ӧ��������336mL���������������ʵ�����������Ԫ���غ��֪n��HCl��=2n��H2��������������������ʵ���Ũ�ȣ�
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼�����
��3����ʵ��֮���������м���NaOH��Һ����ʹ�Ͻ��е���ǡ���ܽ⣬���γɺ����ij�������ʹMg2+�պó�����ȫ���Ͻ��е���Ԫ��ȫ��ת��ΪAlO2-����Ӧ����Һ������Ϊ�Ȼ��ơ�ƫ�����ƣ��ɣ�2���м���Mg��Al�����ʵ�����֪����Al�����ʵ�����������Ԫ���غ����ƫ�����Ƶ����ʵ����������������غ������Һ��n��NaCl��=n��HCl���������������غ��֪n��NaOH��=n��NaCl��+n��NaAlO2����n��Na+��=n��NaOH�����ٸ���V=$\frac{n}{c}$�����������Ƶ������
��� �⣺��1������Ũ�����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ255mg��$\frac{336mL}{280mL}$=306mg�������н���ʣ�࣬����㣬
������ȫ��Ӧ��������336mL�����������ʵ���Ϊ$\frac{0.336L}{22.4L/mol}$=0.015mol��������Ԫ���غ��֪n��HCl��=2n��H2��=2��0.015mol=0.03mol������������ʵ���Ũ��Ϊ$\frac{0.03mol}{0.03L}$=1mol/L��
�ʴ�Ϊ����������������1mol/L��
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ���������֪24x+27y=0.255�����ݵ���ת���غ���2x+3y=$\frac{0.28L}{22.4L/mol}$��2���������̽�ã�x=0.005��y=0.005���ʺϽ���þ���������ʵ���֮��Ϊ0.005mol��0.005mol=1��1��
�ʴ�Ϊ��1��1��
��3����ʵ��֮���������м���NaOH��Һ��ǡ��ʹ�Ͻ��е���Ԫ��ȫ��ת��ΪAlO2-����ʹMg2+�պó�����ȫ����Ӧ����Һ������Ϊ�Ȼ��ơ�ƫ�����ƣ��ɣ�2���м���Mg��Al�����ʵ�����֪����Al�����ʵ���Ϊ0.005mol��$\frac{459mg}{255mg}$=0.009mol��������Ԫ���غ��֪n��NaAlO2��=n��Alԭ�ӣ�=0.009mol�������������غ��֪n��NaCl��=1mol/L��0.03L=0.03mol�������������غ��֪n��NaOH��=n��NaCl��+n��NaAlO2��=0.03mol+0.009mol=0.039mol��n��Na+��=n��NaOH��=0.039mol������Ҫ����������Һ�����Ϊ$\frac{0.039mol}{1mol/L}$=0.039L=39mL��
�ʣ�NaAlO2Ϊ0.009mol��NaClΪ0.03mol������NaOH��Һ���Ϊ39mL��
���� ���⿼������ļ��㣬���ݱ������ݹ�ϵ�жϷ�Ӧ�Ĺ��������ǹؼ�����3����ע�������غ�˼���𣬽Ϻõؿ���ѧ������������������Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  3-��-2-�һ����� | B�� |  2��2-����-4-�һ����� | ||
| C�� |  �ڼ����� | D�� | 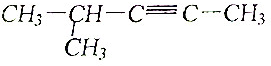 2-��-3-���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ���� | B�� | �ȵ�̼������Һ | C�� | ��ˮ | D�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ѹǿ����ʱ��仯 | |
| B�� | �����ڸ����ʵ�Ũ�Ȳ���ʱ��仯 | |
| C�� | ��λʱ����ÿ����1molX������2molY | |
| D�� | ���������Է�����������ʱ��仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢܢݢ� | B�� | �ڢۢܢ� | C�� | �٢ڢۢܢ� | D�� | �٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
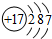
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
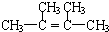 ��
��
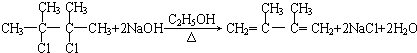 ��C�Ļ�ѧ������2��3-����-1��3-����ϩ����Ӧ�Ļ�ѧ����ʽΪ
��C�Ļ�ѧ������2��3-����-1��3-����ϩ����Ӧ�Ļ�ѧ����ʽΪ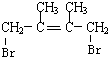 +2NaOH$��_{��}^{H_{2}O}$
+2NaOH$��_{��}^{H_{2}O}$ +2NaBr���ܵķ�Ӧ�����Ǽӳɷ�Ӧ��
+2NaBr���ܵķ�Ӧ�����Ǽӳɷ�Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com