
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��
��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
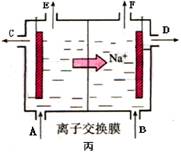
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010������������ʦ���и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ������
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��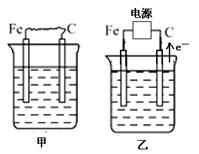

��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������и���9���¿������ۺ����⣨��ѧ���֣� ���ͣ������
(16��)(1)��֪����ԭ��HSO3��>I����������IO3��>I2����NaIO3��Һ�еμ�����NaHSO3��Һ���������з�Ӧ��NaIO3+NaHSO3��I2+Na2SO4+H2SO4+H2O
����ƽ������Ӧ�Ļ�ѧ����ʽ(����ѧ���������ڷ�����)����д������������____________��
����NaIO3��Һ�еμӹ���NaHSO3��Һ����Ӧ��ȫ���ƲⷴӦ����Һ�еĻ�ԭ����Ϊ____________ (�ѧʽ)��
(2)��ij�ܱ������м���0.15 mol/L A��0.05 mol/L C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯����ͼ�м�ͼ��ʾ[t0ʱc(B)δ������t1ʱ����0.05 mol/L]����ͼΪt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�������淴Ӧ������ʱ��仯�������

����t4ʱ�ı������Ϊ��Сѹǿ����B����ʼ���ʵ���Ũ��Ϊ________mol/L��
����t1=15 s����t0��t1����CŨ�ȱ仯��ʾ��ƽ����Ӧ����Ϊv(C)=_______mol/(L��s)��
��t3ʱ�ı��ijһ��Ӧ����������_______(ѡ�����)��
aʹ�ô��� b����ѹǿ c����Ӧ��Ũ��
���мס��������ݻ���Ϊ2L���ܱ��������ڿ����������¶���ͬ�Һ㶨����£������ͨ��3mol A���ﵽƽ��ʱ��B���������Ϊ20���������������г���1 mol C��0.5mol B���ﵽƽ��ʱ��C��Ũ��c(C)=________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ������
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��
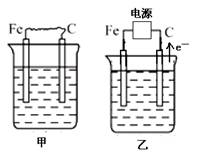
��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)(1)��֪����ԭ��HSO3��>I����������IO3��>I2����NaIO3��Һ�еμ�����NaHSO3��Һ���������з�Ӧ��NaIO3+NaHSO3��I2+Na2SO4+H2SO4+H2O
����ƽ������Ӧ�Ļ�ѧ����ʽ(����ѧ���������ڷ�����)����д������������____________��
����NaIO3��Һ�еμӹ���NaHSO3��Һ����Ӧ��ȫ���ƲⷴӦ����Һ�еĻ�ԭ����Ϊ____________ (�ѧʽ)��
(2)��ij�ܱ������м���0.15 mol/L A��0.05 mol/L C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯����ͼ�м�ͼ��ʾ[t0ʱc(B)δ������t1ʱ����0.05mol/L]����ͼΪt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�������淴Ӧ������ʱ��仯�������
����t4ʱ�ı������Ϊ��Сѹǿ����B����ʼ���ʵ���Ũ��Ϊ________mol/L��
����t1=15 s����t0��t1����CŨ�ȱ仯��ʾ��ƽ����Ӧ����Ϊv(C)=_______mol/(L��s)��
��t3ʱ�ı��ijһ��Ӧ����������_______(ѡ�����)��
aʹ�ô��� b����ѹǿ c����Ӧ��Ũ��
���мס��������ݻ���Ϊ2L���ܱ��������ڿ����������¶���ͬ�Һ㶨����£������ͨ��3mol A���ﵽƽ��ʱ��B���������Ϊ20���������������г���1 mol C��0.5mol B���ﵽƽ��ʱ��C��Ũ��c(C)=________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com