
��11�֣�A��B��C��D��E��F�������������±仯��ϵ��E�ǵ���ɫ��ĩ���жϣ�
��1��д��A��B��C��D��E��F�Ļ�ѧʽ��
B.________��C.________��D.________��E��________��
��2��д���йط�Ӧ�Ļ�ѧ����ʽ(�����ӷ�Ӧ��ֱ��д���ӷ���ʽ)
E��B�� _______________��C��F�� _______________________��F��C�� ________________________��
��3������ʵ�鷽���У�����ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3������������
A. ȡa�˻�����ּ��ȣ�����b��
B. ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹���
C. ȡa�˻����������ϡ�����ַ�Ӧ���ݳ�����ֱ���ü�ʯ�����գ�����b��
D. ȡa�˻����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��塣
��1�� B��NaOH C��Na2CO3 D��NaCl E��Na2O2
��2�� 2Na2O2��2H2O===4Na����4OH����O2����CO ��H2O��CO2===2HCO
��H2O��CO2===2HCO
2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��3�� C
�������������A�������ڵ�ȼʱ��������ɫ��ĩ����A��Na��E��Na2O2��A��ˮ����B����B��NaOH;NaOH��Һ��ͨ��CO2�����C��Na2CO3����Na2CO3��Һ��ͨ�������CO2�����F��NaHCO3;NaHCO3���ȶ������ȷֽ����Na2CO3��Na2CO3��HCl������Ӧ����D��NaCl����1�� A��B��C��D��E��F�Ļ�ѧʽ�ֱ���B��NaOH��C��Na2CO3��D��NaCl��E��Na2O2����2�� E��B�����ӷ���ʽ��2Na2O2��2H2O===4Na����4OH����O2����C��F�����ӷ���ʽ��CO ��H2O��CO2===2HCO
��H2O��CO2===2HCO ��F��C�Ļ�ѧ����ʽ�ǣ�2NaHCO3
��F��C�Ļ�ѧ����ʽ�ǣ�2NaHCO3  Na2CO3��H2O��CO2������3��A. ȡa�˻�����ּ��ȣ�ֻ��NaHCO3��ֽ����Na2CO3��CO2��ˮ�����ص���������CO2��ˮ�������ͣ����Ը��ݼ��������b�˾Ϳ��Լ����NaHCO3����������˾���ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3�������������� B. ���߶��������ᷢ����Ӧ�����õ���������NaCl.����ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a��2x+y=b��58.5��������⣬�õ�Na2CO3������������ͻ�õ�Na2CO3�������������� C. �ڻ�������������ᷢ����Ӧʱ��������CO2��ˮ���������Ա���ʯ�����գ���˲���ȷ��CO2���������Ͳ��ܼ��������и��Ե����������Բ���ȷ��Na2CO3������������ȷ��D.���߶��ܹ���Ba(OH)2������Ӧ����BaCO3����������ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a�� x+y=b��197��������⣬���Լ���õ�Na2CO3������������ͻ�õ�Na2CO3��������������
Na2CO3��H2O��CO2������3��A. ȡa�˻�����ּ��ȣ�ֻ��NaHCO3��ֽ����Na2CO3��CO2��ˮ�����ص���������CO2��ˮ�������ͣ����Ը��ݼ��������b�˾Ϳ��Լ����NaHCO3����������˾���ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3�������������� B. ���߶��������ᷢ����Ӧ�����õ���������NaCl.����ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a��2x+y=b��58.5��������⣬�õ�Na2CO3������������ͻ�õ�Na2CO3�������������� C. �ڻ�������������ᷢ����Ӧʱ��������CO2��ˮ���������Ա���ʯ�����գ���˲���ȷ��CO2���������Ͳ��ܼ��������и��Ե����������Բ���ȷ��Na2CO3������������ȷ��D.���߶��ܹ���Ba(OH)2������Ӧ����BaCO3����������ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a�� x+y=b��197��������⣬���Լ���õ�Na2CO3������������ͻ�õ�Na2CO3��������������
���㣺�������ʵ��ƶϡ�����ʽ����д��������к��еijɷֺ����IJⶨ�������жϵ�֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
10��)��5.6 g Fe���뵽100 mL��ϡ��������Һ�У�ǡ����ȫ��Ӧ��
��1��д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ʒ������Ŀ��
��2���������������ڱ�״���µ������ϡ��������ʵ���Ũ�ȡ�
��3�����㷴Ӧ��ת�Ƶĵ�����Ŀ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и������У������������������Ԫ�صĵ���ֱ�ӻ��ϵõ�����
| A��FeS | B��FeCl2 | C��FeCl3 | D��Fe3O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��7�֣�A��B��Ϊ���ε�ˮ��Һ��A�����ԣ�B�ʼ��Բ����������ԡ�����Ϊ���ʵ�鲽���ʵ������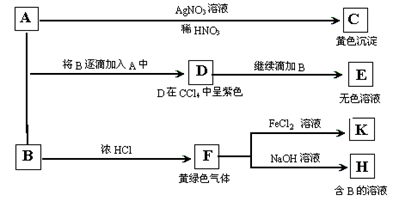
��ش�
��1��д��A��B��C�Ļ�ѧʽ��A__________��B__________��C__________��
��2��д��A��D�����ӷ���ʽ��______________________________________��
��3��д����SO2����ͨ��K��Һ������Ӧ�����ӷ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��10�֣�ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת�� ��ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺
��1��Z��L��Ӧ�������� ��
��2��K�ĵ���ʽΪ ��
��3�� д��B��F�����ӷ���ʽ ��
��4�� д��K��CO2��Ӧ�Ļ�ѧ����ʽ ��
��5�� Y��NaClO��B�Ļ����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�������д���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣�����A����ͼ��ʾ�Ĺ���ת��Ϊ������D��DΪǿ�ᣬ��ش��������⣺
��1����A�ڳ�����Ϊ���嵥����ش�
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________��
�ڽ�Cͨ��ˮ��Һ�У���Ӧ��ѧ����ʽΪ________________________��
����A��B�ڳ�����Ϊ������Ϊ�������ش�
��A�Ļ�ѧʽ�ǣ�A________��
��B����C�Ļ�ѧ����ʽΪ________________________��
��һ��������̼������D��Ӧ�ķ���ʽΪ________________________���÷�Ӧ��D��
������________________.
��3����A�ڳ�����Ϊ���嵥����ش�
��D�Ļ�ѧʽ��________��
����2mol D��Ũ��Һ�м���������Cu���ȣ���״���²������������_______22.4L
������ڡ������ڡ���С�ڡ�����ԭ��Ϊ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����豸����ʱ������ѧ��ת��Ϊ���ܵ���
A����̫���ܵ�� |
B������ӵ�� |
C��̫���ܼ����� |
D��ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��������������������ء������й�˵���У��������
| A�����ø����������ҩ�ý��һ�����彡�����Σ�� |
| B���Ʋ��ƻ���������ȶ�п�������ʴ |
| C���رո��ܺĵĻ�����ҵ�������ˡ���̼���á�����ּ |
| D��������Ҫ���ɻ�ʯȼ��ȼ���ŷŵĶ�������������̳��Լ�������β���������ﳾ�ȵ��µ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com