
��14�֣�ij��ѧ��ȤС��Ϊ��̽�����ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֡���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������롣
[�������]
����1�� ������2 ������3 ��
Ϊ����֤�²⣬��С�����ʵ�����̽����
[ʵ��̽��]
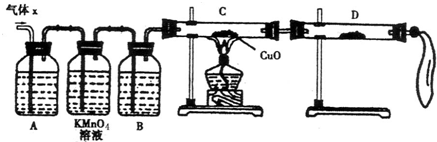
��С��ͬѧ����ͼ��ʾװ�ã��������a��ͨ�룺
��1��B��Ӧ��װ���� �Լ������ţ���
A��NaCl��Һ B��KMnO4��Һ C��Ũ��ˮ D������NaHCO3��Һ
��2��A��Ʒ��������ǣ� ��
��3��D�г���ʯ��ˮ�������ǣ� ��
ͨ����ʵ�飬С��ͬѧ�۲쵽��������ʵ������
��A��Ʒ����ɫ ��C��Ʒ�첻��ɫ ��D�г���ʯ��ˮ�����
[����]
����������С��ͬѧȷ�ϸ�����ijɷ��� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C��ʯī�� | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮
ij��ѧ��ȤС��Ϊ��̽���ڳ�����ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֱ���ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com