

| A£® | µē½ā¹ż³ĢÖŠ£¬a µē¼«±ķĆęĻČÓŠŗģÉ«ĪļÖŹĪö³ö£¬ŗóÓŠĘųÅŻ²śÉś | |
| B£® | b µē¼«ÉĻ·¢Éś·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ4OH--4e-=2H2O+O2”ü | |
| C£® | ĒśĻß O”«P ¶Ī±ķŹ¾ O2 µÄĢå»ż±ä»Æ | |
| D£® | “ÓæŖŹ¼µ½ Q µćŹ±ŹÕ¼Æµ½µÄ»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæĪŖ 12 g/mol |
·ÖĪö ÓÉĶ¼æÉÖŖ£¬µēĮ÷ÓÉÕż¼«Į÷Ļņøŗ¼«£¬ŌņbĪŖŃō¼«£¬aĪŖŅõ¼«£¬¶čŠŌµē¼«µē½āŅ»¶ØĮæµÄĮņĖįĶČÜŅŗ£¬·¢Éś2CuSO4+2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2Cu+O2”ü+2H2SO4£¬½įŗĻĶ¼2æÉÖŖ£¬Ķعż0.2molµē×ÓŹ±µē½āĮņĖįĶ£¬Č»ŗóµē½āĮņĖįČÜŅŗ£¬·¢Éś2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£¬Pµ½QµćŹ±ŹÕ¼Æµ½µÄ»ģŗĻĘųĢåĪŖĒāĘųŗĶŃõĘų£¬ŅŌ“ĖĄ“½ā“š£®
½ā“š ½ā£ŗÓÉĶ¼æÉÖŖ£¬µēĮ÷ÓÉÕż¼«Į÷Ļņøŗ¼«£¬ŌņbĪŖŃō¼«£¬aĪŖŅõ¼«£¬¶čŠŌµē¼«µē½āŅ»¶ØĮæµÄĮņĖįĶČÜŅŗ£¬·¢Éś2CuSO4+2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2Cu+O2”ü+2H2SO4£¬½įŗĻĶ¼2æÉÖŖ£¬Ķعż0.2molµē×ÓŹ±µē½āĮņĖįĶ£¬Č»ŗóµē½āĮņĖįČÜŅŗ£¬·¢Éś2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£¬
A£®aĪŖŅõ¼«£¬ĻČ·¢ÉśCu2++2e-ØTCu£¬ŗó·¢Éś2H++2e-ØTH2”ü£¬aµē¼«±ķĆęĻČÓŠŗģÉ«ĪļÖŹĪö³ö£¬ŗóÓŠĘųÅŻ²śÉś£¬¹ŹAÕżČ·£»
B£®bĪŖŃō¼«£¬ČÜŅŗÖŠµÄĒāŃõøłĄė×ӷŵē£¬Ōņbµē¼«ÉĻ·¢ÉśµÄ·“Ó¦·½³ĢŹ½ĪŖ£ŗ4OH--4e-ØTH2O+O2”ü¹ŹBÕżČ·£»
C£®ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬ĒśĻß0”«P¶Ī±ķŹ¾O2µÄĢå»ż±ä»Æ£¬ĒśĻßP”«Q¶Ī±ķŹ¾H2ŗĶO2»ģŗĻĘųĢåµÄĢå»ż±ä»Æ£¬¹ŹCÕżČ·£»
D£®ĒśĻß0”«P¶Ī±ķŹ¾O2µÄĢå»ż±ä»Æ£¬Pµć1.12LĪŖO2£¬ĘäĪļÖŹµÄĮæĪŖ0.05mol£¬PQ¶Ī3.36LĘųĢåÖŠ£¬Óɵē½āĖ®·“Ó¦æÉÖŖ0.2molµē×ÓĶعżŹ±Éś³É0.1mol H2”¢0.05mol O2£¬Ōņ“ÓæŖŹ¼µ½QµćŹÕ¼Æµ½µÄ»ģŗĻĘųĢåÖŠO2ĪŖ0.1mol£¬HĪŖ0.1mol£¬¹Ź»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæĪŖ$\frac{0.1mol”Į32g/mol+0.1mol”Į2g/mol}{0.1mol+0.1mol}$=17g•mol-1£¬¹ŹD“ķĪó£®
¹ŹŃ”£ŗD£®
µćĘĄ ±¾Ģāæ¼²éµē½āŌĄķ£¬Ć÷Č·Ķ¼ĻóÕāµē×Ó×ŖŅĘÓėÉś³ÉĘųĢåµÄ¹ŲĻµ¼°Ąė×ӵķŵēĖ³ŠņŹĒ½ā“š±¾ĢāµÄ¹Ų¼ü£¬ŹģĻ¤µē½āŌĄķ¼“æɽā“š£¬ĢāÄæÄŃ¶Č²»“ó£®


 æ¼Ē°±ŲĮ·ĻµĮŠ“š°ø
æ¼Ē°±ŲĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | K+”¢Fe3+”¢SCN-”¢Cl- | B£® | Fe3+”¢K+”¢OH-”¢SO42- | ||
| C£® | Fe2+”¢K+”¢Cl-”¢SO42- | D£® | Na+”¢H+”¢SO42-”¢CO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²»É÷½«ÅؼīČÜŅŗÕ“µ½Ę¤·ōÉĻ£¬ŅŖĮ¢¼“ÓĆ“óĮæĖ®³åĻ“£¬Č»ŗóĶæÉĻÅšĖį | |
| B£® | øųŹŌ¹ÜĄļµÄŅŗĢå¼ÓČČ£¬ŅŗĢåµÄĢå»żŅ»°ć²»³¬¹żŹŌ¹ÜČŻ»żµÄ$\frac{2}{3}$ | |
| C£® | ¾Ę¾«×Å»šŹ±æÉÓĆĖ®ĘĖĆš | |
| D£® | ÅäÖĘĮņĖįČÜŅŗŹ±£¬æÉĻČŌŚĮæĶ²ÖŠ¼ÓČėŅ»¶ØĢå»żµÄĖ®£¬ŌŁŌŚ½Į°čĢõ¼žĻĀĀżĀż¼ÓČėÅØĮņĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 10 s | B£® | 12 s | C£® | “óÓŚ12 s | D£® | Š”ÓŚ12 s |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Įņ“śĮņĖįÄĘ£ØNa2S2O3•5H2O£©Ė×Ćū”°“óĖÕ“ņ”±£¬ÓÖ³ĘĪŖ”°ŗ£²Ø”±£¬æÉÓĆÓŚÕÕĻąŅµ×÷¶ØÓ°¼Į£¬Ņ²æÉÓĆÓŚÖ½½¬ĘÆ°××÷ĶŃĀČ¼ĮµČ£®ĖüŅ×ČÜÓŚĖ®£¬ÄŃČÜÓŚŅŅ“¼£¬¼ÓČČ”¢Ņ×·Ö½ā£¬ŌŚĖįŠŌČÜŅŗÖŠ²»ÄÜĪČ¶Ø“ęŌŚ£®¹¤ŅµÉĻ³£ÓĆŃĒĮņĖįÄĘ·Ø”¢Įņ»Æ¼ī·ØµČÖʱø£®Ä³ŹµŃéŹŅÄ£Äā¹¤ŅµĮņ»Æ¼ī·ØÖĘČ”Įņ“śĮņĖįÄĘ£¬Ę䷓ӦװÖĆ¼°ĖłŠčŹŌ¼ĮČēĶ¼£ŗ
Įņ“śĮņĖįÄĘ£ØNa2S2O3•5H2O£©Ė×Ćū”°“óĖÕ“ņ”±£¬ÓÖ³ĘĪŖ”°ŗ£²Ø”±£¬æÉÓĆÓŚÕÕĻąŅµ×÷¶ØÓ°¼Į£¬Ņ²æÉÓĆÓŚÖ½½¬ĘÆ°××÷ĶŃĀČ¼ĮµČ£®ĖüŅ×ČÜÓŚĖ®£¬ÄŃČÜÓŚŅŅ“¼£¬¼ÓČČ”¢Ņ×·Ö½ā£¬ŌŚĖįŠŌČÜŅŗÖŠ²»ÄÜĪČ¶Ø“ęŌŚ£®¹¤ŅµÉĻ³£ÓĆŃĒĮņĖįÄĘ·Ø”¢Įņ»Æ¼ī·ØµČÖʱø£®Ä³ŹµŃéŹŅÄ£Äā¹¤ŅµĮņ»Æ¼ī·ØÖĘČ”Įņ“śĮņĖįÄĘ£¬Ę䷓ӦװÖĆ¼°ĖłŠčŹŌ¼ĮČēĶ¼£ŗ| ±ąŗÅ | 1 | 2 | 3 | 4 |
| ĻūŗÄNa2S2O3±ź×¼ČÜŅŗµÄĢå»ż/mL | 18.02 | 17.98 | 18.00 | 20.03 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
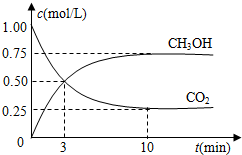
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ¢Ł | H2£Øg£©+$\frac{1}{2}$O2£Øg£©ØTH2O£Øg£©”÷H=-242kJ/mol£» |
| ¢Ś | 2H2£Øg£©+O2£Øg£©ØT2H2O£Øl£©”÷H=-572kJ/mol£» |
| ¢Ū | C£Øs£©+$\frac{1}{2}$O2£Øg£©ØTCO£Øg£©”÷H=-110.5kJ/moL£» |
| ¢Ü | C£Øs£©+O2£Øg£©ØTCO2£Øg£©”÷H=-393.5kJ/moL£» |
| ¢Ż | CO2£Øg£©+2H2O£Øg£©ØTCH4£Øg£©+2O2£Øg£©”÷H=+802kJ/moL |
| »Æѧ¼ü | O=O | C-C | H-H | O-O | C-O | O-H | C-H |
| ¼üÄÜkJ/mol | 497 | 348 | 436 | 142 | 351 | 463 | 414 |
| A£® | H2Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖH2£Øg£©+$\frac{1}{2}$O2£Øg£©ØTH2O£Øg£©”÷H=-242kJ/mol | |
| B£® | ČČ»Æѧ·½³ĢŹ½£ŗC£Øs£©+H2O£Øg£©?H2£Øg£©+CO£Øg£©”÷H=+175.5kJ/moL | |
| C£® | CH4µÄČ¼ÉÕČČ”÷H=-802kJ/moL | |
| D£® | ¹ĄĖć³öC=O¼üÄÜĪŖ800kJ/moL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū | B£® | ¢Ū¢Ü¢Ż | C£® | ¢Ś¢Ü¢Ż | D£® | ¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀ³£Ń¹ĻĀ£¬11.2LĀČĘųĖłŗ¬µÄŌ×ÓŹżÄæĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬1.12LO2ŗĶ1.12 L CO2¾łŗ¬ÓŠ0.1NAŃõŌ×Ó | |
| C£® | ±ź×¼×“æöĻĀ£¬22.4LæÕĘųŗ¬ÓŠNAøöµ„ÖŹ·Ö×Ó | |
| D£® | ±ź×¼×“æöĻĀ£¬22.4LH2Oŗ¬ÓŠNAøöĖ®·Ö×Ó |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com