
ʵ������50 mL 0.50 mol·L��1���ᡢ50 mL 0.55 mol·L��1 NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
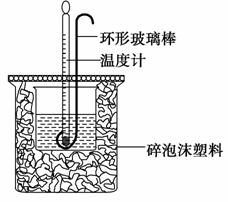
������������⣺
(1)ʵ��ʱ�û��β�����������Һ�ķ�����________________________________________________________________
_______________________________________________________________��
������ͭ˿��������滷�β�������������_______________________________________________________________��
(2)�����ݴ�����t2—t1��3.4 �档���ʵ���õ��к��Ȧ�H��________[�����NaOH��Һ���ܶȰ�1 g·cm��3���㣬��Ӧ������Һ�ı�����(c)��4.18 J·(g·��)��1����]��
(3)����NaOH��Һ��Ϊ��ͬ�������ͬŨ�ȵİ�ˮ������к���Ϊ��H1����H1�릤H�Ĺ�ϵΪ����H1________��H(�����������������)��������________________________________________________________________��
������(1)���ڱ�ʵ�飬���������ƺ����ᾡ���ܵ���ȫ��Ӧ�Ǽ�С����һ�����棬����ʵ��ʱ�û��β��������½������Է����¶ȼ�����������ɢʧ�Ǽ�С������һ����Ҫ���棬����ѡ�ò�������������ͭ˿��
(2)��H����[100 g��4.18��10��3 kJ·(g·��)��1��3.4 ��]��0.025 mol����56.8 kJ·mol��1
(3)��������ʵĵ�����������ȵģ���NaOH��Һ��Ϊ��ͬ�������ͬŨ�ȵİ�ˮ��Ӧ��ų��������٣����Ԧ�H1����H��
�𰸡�(1)���½���(���������)��Cu���ȿ죬��ֹ������ʧ��(2)��56.8 kJ·mol��1��(3)����NH3·H2O������������ʣ���������


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������ж����ڼ��Է��ӵ�һ����(����)
A��HF��NH3��O3 B��NO��SO2��CCl4
C��SO3��H2O��N2 D��CO��BF3��CS2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A�������£�ij��Һ����ˮ�������c(H��)��1��10��amol·L��1����a>7ʱ�������Һ��pHһ��Ϊ14��a
B��pH��9��CH3COONa��Һ��pH��9��NH3·H2O��Һ������Һ��ˮ�ĵ���̶���ͬ
C���¶�һ��ʱ����ˮ�еμ����������γ�ϡ��Һ��ˮ�����ӻ�����Kw����
D�������£���pH��4�Ĵ�����Һϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������[Zn(NH3)4]2����
�ش��������⣺
(1)��������������������Һ����Һ����Ԫ�صĴ�����ʽΪ________________(�û�ѧʽ��ʾ)��
(2)д��п������������Һ��Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
(3)���и����е�������Һ������μӵ�ʵ�鷽�����ɼ������________________��
�����������������ơ����������Ͱ�ˮ��������п���������ơ�������п�Ͱ�ˮ
(4)д�������������백ˮ��Ӧ�����ӷ���ʽ��________________________________________________________________________________________________________________________________________________��
�Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��_____________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ̽��NaHCO3��Na2CO3������(��������Ũ�Ⱦ�Ϊ1 mol·L��1)��Ӧ�����е���ЧӦ��ʵ�����������ݣ�
| ��� | 35 mL�Լ� | ���� | ���ǰ �¶�/�� | ��Ϻ� �¶�/�� |
| �� | ˮ | 2.5 g NaHCO3 | 20.0 | 18.5 |
| �� | ˮ | 3.2 g Na2CO3 | 20.0 | 24.3 |
| �� | ���� | 2.5 g NaHCO3 | 20.0 | 16.2 |
| �� | ���� | 3.2 g Na2CO3 | 20.0 | 25.1 |
�ɴ˵ó��Ľ�����ȷ���� (����)��
A��Na2CO3��Һ������ķ�Ӧ�����ȷ�Ӧ
B��NaHCO3��Һ������ķ�Ӧ�Ƿ��ȷ�Ӧ
C��20.0 ��ʱ����3.2 g Na2CO3�ı�����Һ��35 mL�����Ϻ���¶Ƚ�����25.1 ��
D��20.0 ��ʱ����2.5 g NaHCO3�ı�����Һ��35 mL�����Ϻ���¶Ƚ���
��16.2 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ��ʽ��ͼ��ʾ������������ȷ���� (����)��
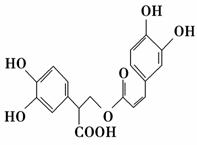
A���Ե��������嵥��ֻ�ܷ���ȡ����Ӧ
B��1 mol�Ե���������ܺ�9 mol���������ӳɷ�Ӧ
C���Ե�������Է���ˮ�ⷴӦ��ȡ����Ӧ��������Ӧ
D��1 mol�Ե������������5 mol NaOH������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A(C11H8O4)������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��
�ش��������⣺
(1)B�ķ���ʽΪC2H4O2��������ֻ��һ�������š���B�Ľṹ��ʽ��________��B���Ҵ���Ũ������¼��ȷ�Ӧ����D���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�÷�Ӧ��������________��д�������ܷ���������Ӧ��B��ͬ���칹��Ľṹ��ʽ________________________________________________________________________��
(2)C�Ƿ��㻯�����Է�������Ϊ180����̼����������Ϊ60.0%�������������Ϊ4.4%������Ϊ������C�ķ���ʽ��________��
(3)��֪C�ķ�����������ȡ����������һ��ȡ������֧�����Һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ż�����̼��������Һ��Ӧ�ų�����Ĺ����ţ����ȡ�����ϵĹ�����������________����������ȡ������ͬ���ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ��________________________________________________________________________��
(4)A�Ľṹ��ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ĵ��ӽṹ�У���һ��������С��ԭ�ӿ�����(����)
A��ns2np3 B��ns2np5 C��ns2np4 D��ns2np6
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com