
| |||||||||||||||||||
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

| ||
| ||
| ||
| ”÷ |
| ||
| ”÷ |
| øßĪĀ”¢øßŃ¹ |
| “߻ƼĮ |
| øßĪĀ”¢øßŃ¹ |
| “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¹¤ŅµÉś²śµÄ“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClŌÓÖŹ£®Ä³Š£ŃŠ¾æŠŌѧĻ°»ī¶ÆŠ”×éĪŖĮĖ²ā¶Ø»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£¬ÄāŹ¹ÓĆČēĶ¼ŹµŃé×°ÖĆ£ØĖµĆ÷£ŗĮ¬½Ó¼×ŗĶŅŅµÄĻšĘ¤¹ÜÓŠĢś¼ŠæŲÖĘ£©£¬ĻČ²ā¶ØŅ»¶ØĮæµÄѳʷŗĶĖį·“Ó¦·Å³ö¶žŃõ»ÆĢ¼µÄÖŹĮ棬ŌŁ¼ĘĖć»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£®
¹¤ŅµÉś²śµÄ“æ¼īÖŠ³£ŗ¬ÓŠÉŁĮæµÄNaClŌÓÖŹ£®Ä³Š£ŃŠ¾æŠŌѧĻ°»ī¶ÆŠ”×éĪŖĮĖ²ā¶Ø»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£¬ÄāŹ¹ÓĆČēĶ¼ŹµŃé×°ÖĆ£ØĖµĆ÷£ŗĮ¬½Ó¼×ŗĶŅŅµÄĻšĘ¤¹ÜÓŠĢś¼ŠæŲÖĘ£©£¬ĻČ²ā¶ØŅ»¶ØĮæµÄѳʷŗĶĖį·“Ó¦·Å³ö¶žŃõ»ÆĢ¼µÄÖŹĮ棬ŌŁ¼ĘĖć»ģŗĻĪļÖŠ“æ¼īµÄÖŹĮæ·ÖŹż£®| 53(w-m) |
| 22n |
| 53(w-m) |
| 22n |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

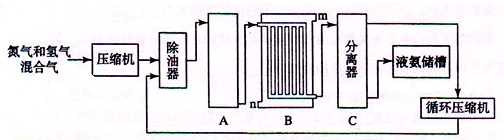
Ķ¼1-9
£ØA£©ŌŚøÉŌļ¹ÜÄŚĢīĀś¼īŹÆ»Ņ£¬×ÜÖŹĮæĪŖm g£»
£ØB£©Č”n gѳʷװČė¹ćæŚĘæÖŠ£»
£ØC£©¼ģŃé×°ÖƵÄĘųĆÜŠŌ£»
£ØD£©»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬ŌŁ³ĘøÉŌļ¹Ü×ÜÖŹĮæĪŖw g£»
£ØE£©¹Ų±ÕÖ¹Ė®¼Š£»
£ØF£©“ņæŖÖ¹Ė®¼Š£»
£ØG£©»ŗ»ŗ¼ÓČėĻ”ĮņĖįÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹£»
£ØH£©»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ”£
£Ø1£©ÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£ØĢīŠ“ŠņŗÅ£©£ŗC”ś_______”śF________”śE_______”śG________”śD”£
£Ø2£©²Ł×÷²½ÖčDÖŠ£¬ŅŖ»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬¹ÄČėæÕĘųµÄ×÷ÓĆŹĒ________________£»×°ÖĆ¼×µÄ×÷ÓĆŹĒ________________£»×°ÖĆŅŅµÄ×÷ÓĆŹĒ________________”£
£Ø3£©¼ĘĖćČÜŅŗÖŠNa2CO3ÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ_____________________________________”£
£Ø4£©ČōČ„µō×°ÖĆ¼×£¬²ā¶Ø½į¹ū»į________£»ČōČ„µō×°ÖĆŅŅ£¬²ā¶Ø½į¹ū»į________”££ØĢī”°Ę«“ó”±”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗ£ÄĻ ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
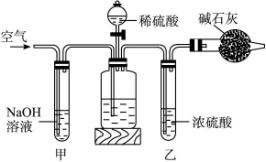
A.ŌŚøÉŌļ¹ÜÄŚĢīĀś¼īŹÆ»Ņ£¬ÖŹĮæĪŖm g”£
B.Č”n gѳʷװČė¹ćæŚĘæÖŠ”£
C.¼ģŃé×°ÖƵÄĘųĆÜŠŌ”£
D.»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬ŌŁ³ĘøÉŌļ¹ÜÖŹĮæĪŖw g”£
E.¹Ų±ÕÖ¹Ė®¼Š”£
F.“ņæŖÖ¹Ė®¼Š”£
G.»ŗ»ŗ¼ÓČėĻ”ĮņĖįÖĮ²»ŌŁ²śÉśĘųĢåĪŖÖ¹”£
H.»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ”£
£Ø1£©ÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£ØĢīŠ“·ūŗÅ£©£ŗ
C”ś________E”ś________”ś________”ś________”śG”śE”śD
£Ø2£©²Ł×÷²½ÖčDÖŠ£¬ŅŖ»ŗ»ŗ¹ÄČėæÕĘųŹż·ÖÖÓ£¬¹ÄČėæÕĘųµÄ×÷ÓĆŹĒ__________£»×°ÖĆ¼×µÄ×÷ÓĆŹĒ__________£»×°ÖĆŅŅµÄ×÷ÓĆŹĒ______________________________”£
£Ø3£©¼ĘĖćČÜŅŗÖŠNa2CO3ÖŹĮæ·ÖŹżµÄ¼ĘĖćŹ½ĪŖ______________________________”£
£Ø4£©ČōČ„µō×°ÖĆ¼×£¬²ā¶Ø½į¹ū»į£»ČōČ„µō×°ÖĆŅŅ£¬²ā¶Ø½į¹ū»į__________”££ØĢī”°Ę«“ó”±”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com