
��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ������������ͼ��ʾ��

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��ת����������ͼ��ʾ����ش����������⣺

��1��CH3COOOH��Ϊ�������ᣬд������һ����; ��
��2��д��B��E��CH3COOOH��H2O�Ļ�ѧ����ʽ ��
��3��д��F���ܵĽṹ��ʽ ��
��4��д��A�Ľṹ��ʽ ��
��5��1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮���� ��
��6��д��D�������ᣨ���廯�ƺ�Ũ����Ļ������ȷ�Ӧ�Ļ�ѧ����ʽ��
��
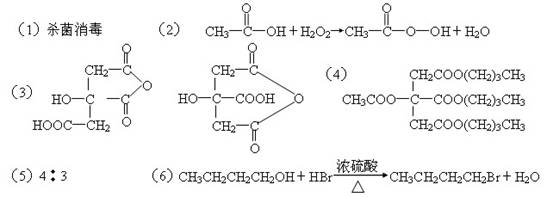
����Ĺؼ����������ʵķ�����ɣ���Ϸ�Ӧ��������Ϣ�ƶ����ʵĽṹ��
��1�������������ǿ�����ԣ�������ɱ��������
��2���Ƚ�B�ķ���ʽ��C2H4O2�������ɵĹ�������ķ���ʽ��C2H4O3��������ȷ��B��E�����ˣ�������E�ķ���ʽӦ�ñ����ɵ�H2OҪ��һ��O������EΪH2O2 ��
��3���Ա�C��F�ķ�����ɣ�����ȷ����C����F�ķ�Ӧ��һ����ˮ��Ӧ��C�к���������COOH����������Ϣ�������÷�Ӧ���̿����ǡ�COOH֮�����ˮ����C�е�������COOH�����������ڵȼ�λ�����ԡ�COOH��������ˮ��ʽ�����ɵ�F�����ֽṹ��
��4���ӷ�����ɿ���D�DZ���һԪ����������������������֧���ı���һԪȩG������D�ķ��ӽṹ��Ҳû��֧����D��1��������
A�������������ܹ�����B��C��D��B��C��D��ֻ����OH����COOH�����ֹ����ţ�AӦ������������A��B��C��D�ķ�����ɿ���ȷ��A�к����ĸ�������B�ġ�COOH��C�ġ�OH�γ���һ������B��������COOH��ֱ�������D�����еġ�OH�γ���������
��5��1molC��1mol��OH��3mol��COOH���ֱ�������Ľ���Na��NaOH��Ӧʱ����������Na��4mol��NaOH��3mol��
��6��D��HBr�ķ�Ӧ�Ǵ��еġ�OH����Brȡ��������±������
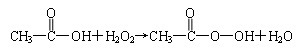



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;__________________________��
(2)д��B+E![]() CH3COOOH+H2O�Ļ�ѧ����ʽ__________________________����֪��ȩ����������Ҳ�����Ƶù������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ______________________��
CH3COOOH+H2O�Ļ�ѧ����ʽ__________________________����֪��ȩ����������Ҳ�����Ƶù������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ______________________��
(3)д��F���ܵĽṹ��ʽ______________________________��
(4)д��A�Ľṹ��ʽ_______________________________��
(5)1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;________________________��
(2)д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ______________________________________
(3)д��F���ܵĽṹ��ʽ_____________________________________________��
(4)д��A�Ľṹ��ʽ_____________________________________________��
(5)1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH�����ʵ���֮����____��
(6)д��D��������(���廯�ƺ�Ũ����Ļ����)���ȷ�Ӧ�Ļ�ѧ����ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ����
��ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D������ͼ���� 
��1��CH3COOOH��Ϊ�������ᣬд������һ����;______________________________��
��2��д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ_____________________________��
��3��д��F���ܵĽṹ��ʽ________________________________��
��4��1Ħ��C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮����__________��
��5��д��D�������ᣨ���廯�ƺ�ŨH2SO4�Ļ������ȷ�Ӧ�Ļ�ѧ����ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 12�� ) ��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ������������:

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

( 1 ) CH3COOOH��Ϊ�������ᣬд������һ����; _________ ��
( 2 ) д�� B + E ![]() CH3COOOH + H2O �Ļ�ѧ����ʽ ____________________ ��
CH3COOOH + H2O �Ļ�ѧ����ʽ ____________________ ��
( 3 ) д��F���ܵĽṹ��ʽ __________________ ��
( 4 ) д��A�Ľṹ��ʽ ______________________ ��
( 5 ) 1 mol C�ֱ�������Ľ���Na��NaOH��Ӧ������Na��NaOH���ʵ���֮���� _____ ��
( 6 ) д��D�������� ( ���廯�ƺ�Ũ����Ļ���� ) ���ȷ�Ӧ�Ļ�ѧ����ʽ:
______________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��5 4.3ȩ��������ϰ���������棩 ���ͣ������
��֪�����Ȼ�֮����Ũ������������ȥһ����ˮ�����������磺

ij�������A�ǹ㷺ʹ�õ��������ܼ���A�������������ܹ�����B��C��D��

(1)CH3COOOH��Ϊ�������ᣬд������һ����;_______________��
(2)д��B+E��CH3COOOH+H2O�Ļ�ѧ����ʽ____________________��
(3)д��F���ܵĽṹ��ʽ____________________��
(4)д��A�Ľṹ��ʽ________________________��
(5)1 mol C�ֱ�������Ľ���Na\,NaOH��Ӧ������Na��NaOH���ʵ���֮����_______��
(6)д��D��������(���廯�ƺ�Ũ����Ļ����)���ȷ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com