��������1��������Ӧ��2Al+2NaOH+2H
2O=2NaAlO
2+3H
2�������ݷ���ʽ����Al����������������Mg���������ټ���Ͻ���Mg������������
��2��Mg���ڿ�����ȼ������MgO��Mg
3N
2�����ݵ�ԭ���غ����n��Mg
3N
2������������m��Mg
3N
2���������ᷴӦ�����Ȼ�þ���Ȼ�泥������������غ����n��MgCl
2�����ٸ���Mgԭ���غ����n��MgO������������m��MgO����
��3��18.2g����̼��þ��Ʒ��������ȫ�ֽ���8.0g ����þ���壬�ų�3.36L������̼����״������������̼������Ϊ
��44g/mol=6.6g����8g+6.6g=14.6g��18.2g������ˮ���ɣ�������ˮ������Ϊ18.2g-14.6g=3.6g������̼ԭ���غ����n��CO
32-��������Mgԭ���غ����n��Mg
2+�������ݵ���غ��жϼ���n��OH
-�����ٸ���HԪ���غ��жϼ���n��H
2O��������ȷ���仯ѧʽ��
��4�������Ӳ���þ��ˮ��ʯ����������̼������ӽ��������ݵ���غ���Լ���n��Cl
-������ȫ���������ڸ�������ȫ�ֽ⣬�õ���������������壬Ӧ������þ���������������̼�����������������ϡ������ȫ�ܽ���ټ���NaOH��Һֱ�����������յõ�4.64g��ɫ����ΪMg��OH��
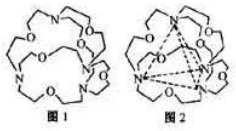
2���ݴ˼���n��Mg
2+�����ɽṹʾ��ͼ��֪��Mg
2+��Al
3+�ĸ�����Ϊ2��1���ݴ˼���n��Al
3+�����ٸ��ݵ���غ����n��OH
-�������������غ��жϼ���n��H
2O��������ȷ��Mg-Al-Cl LDHs�Ļ�ѧʽ��
���
�⣺��1�����������ʵ���Ϊ
=0.3mol��������Ӧ��2Al+2NaOH+2H
2O=2NaAlO
2+3H
2������֪Al�����ʵ���Ϊ0.3mol��
=0.2mol��Al������Ϊ0.2mol��27g/mol=5.4g��Mg������Ϊ10g-5.4g=4.6g���ʺϽ���Mg����������Ϊ
��100%=46%���ʴ�Ϊ��46%��
��2��Mg���ڿ�����ȼ������MgO��Mg
3N
2�����ݵ�ԭ���غ�n��Mg
3N
2��=
n��NH
3��=
��
=0.003mol����m��Mg
3N
2��=0.003mol��100g/mol=0.3g��ȼ�ղ��������ᷴӦ�����Ȼ�þ���Ȼ�泥������������غ�2n��MgCl
2��+n��NH
4Cl��=n��HCl������2n��MgCl
2��+
=0.05L��1.6mol/L����n��MgCl
2��=0.037mol������Mgԭ���غ�n��MgO��+3n��Mg
3N
2��=n��MgCl
2������n��MgO��=0.037mol-0.003mol��3=0.028mol����m��MgO��=0.028mol��40g/mol=1.12g��
�ʴ�Ϊ��MgO 1.12g��Mg
3N
2 0.3g��
��3��18.2g����̼��þ��Ʒ��������ȫ�ֽ���8.0g ����þ���壬�ų�3.36L������̼����״������������̼������Ϊ
��44g/mol=6.6g����8g+6.6g=14.6g��18.2g������ˮ���ɣ�������ˮ������Ϊ18.2g-14.6g=3.6g��
�ų�CO
2�����ʵ���Ϊ
=0.15 mol����n��CO
32-��=0.15 mol��
������n��Mg
2+��=n��MgO��=
=0.2 mol��
��������ݵ���غ㣬��n��OH
-��=2n��Mg
2+��-2n��CO
32-��=0.1mol��
��n��H
2O��=��18.2g-0.1mol��24g/mol-0.15mol��60g/mol-0.1mol�w17g/mol����18g/mol=0.15 mol��
��n��MgCO
3����n[Mg��OH��
2]��n��H
2O��=0.15mol����0.2mol-0.15mol����0.15mol=3��1��3��
�ɵó�����Ļ�ѧʽΪ3MgCO
3?Mg��OH��
2?3H
2O��
�𣺾���Ļ�ѧʽΪ3MgCO
3?Mg��OH��
2?3H
2O��
��4�������Ӳ���þ��ˮ��ʯ����������̼������ӽ��������ݵ���غ�n��Cl
-��=2n��CO
32-��=0.02mol��2=0.04mol��
��ȫ���������ڸ�������ȫ�ֽ⣬�õ���������������壬Ӧ������þ���������������̼�����������������ϡ������ȫ�ܽ���ټ���NaOH��Һֱ�����������յõ�4.64g��ɫ����ΪMg��OH��
2����n��Mg
2+��=n[Mg��OH��
2]=
=0.08mol��
�ɽṹʾ��ͼ��֪��Mg
2+��Al
3+�ĸ�����Ϊ2��1��n��Al
3+��=
n��Mg
2+��=0.04mol��
���ݵ���غ�n��OH
-��=0.04mol��3+0.08mol��2-0.04mol��1=0.24mol��
���������غ���n��H
2O��=��10.66g-0.04mol��27g/mol-0.08mol��24g/mol-0.04mol��35.5g/mol-0.24mol��17g/mol����18g/mol=0.12mol��
��n��Al
3+����n��Mg
2+����n��OH
-����n��Cl
-����n��H
2O��=0.04��0.08��0.24��0.04��0.12=1��2��6��1��3��
��þ��ˮ��ʯ�Ļ�ѧʽΪMg
2Al��OH��
6Cl?3H
2O��
��þ��ˮ��ʯ�Ļ�ѧʽΪMg
2Al��OH��
6Cl?3H
2O��

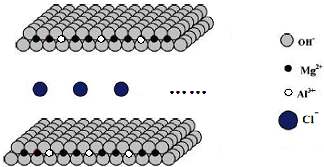 þ���仯�����ڹ�ҵ���й㷺��Ӧ�ã�
þ���仯�����ڹ�ҵ���й㷺��Ӧ�ã�

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�
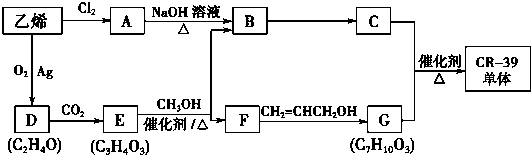
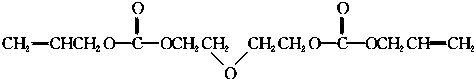
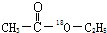 �ĺϳ�·�ߣ��ϳ�·������ͼʾ�����£�CH2-CH2
�ĺϳ�·�ߣ��ϳ�·������ͼʾ�����£�CH2-CH2