
ijŹŠÄāĶ¶×Ź½ØÉčŅ»øö¹¤Ņµ¾Ę¾«³§£¬ÄæµÄŹĒÓĆ¹¤Ņµ¾Ę¾«ÓėĘūÓĶ»ģŗĻÖĘ³É”°ŅŅ“¼ĘūÓĶ”±£¬ŅŌ½ŚŹ”ŹÆÓĶ׏Ō“£¬¼õÉŁ“óĘųĪŪČ¾”£ŅŃÖŖÖĘ¾ĘµÄ·½·ØÓŠČżÖÖ£ŗ
¢ŁŌŚ“߻ƼĮ×÷ÓĆĻĀŅŅĻ©ÓėĖ®·“Ó¦
¢ŚCH3CH2Br+H2O ![]() CH3CH2OH+HBr
CH3CH2OH+HBr
¢Ū£ØC6H10O5£©n£Øµķ·Ū£©+nH2O ![]() nC6H12O6£ØĘĻĢŃĢĒ£©
nC6H12O6£ØĘĻĢŃĢĒ£©
C6H10O6£ØĘĻĢŃĢĒ£©![]() 2C2H5OH+2CO2”ü
2C2H5OH+2CO2ӟ
£Ø1£©·½·Ø¢Ł·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
·½·Ø¢ŚµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ”£
£Ø2£©ĪŖ»ŗ½āŹÆÓĶ¶Ģȱ“ųĄ“µÄÄÜŌ“Ī£»ś£¬ÄćČĻĪŖøĆŹŠÓ¦Ń”ÓĆÄÄŅ»ÖÖ·½·ØÉś²ś¹¤Ņµ¾Ę¾«£æĒė¼ņŹöĄķÓÉ
ӣ
£Ø3£©Čē¹ū“ÓĀĢÉ«»Æѧ£Ø”°Ō×ÓĄūÓĆĀŹ”±×ī“ó»Æ£©µÄ½Ē¶Čæ“£¬ÖĘ¾Ę¾«×īŗƵÄŅ»×é·½·ØŹĒ £ØĢīŠņŗÅ£©
A£®¢Ł B£®¢Ū C£®¢Ł¢Ū D£®¢Ł¢Ś¢Ū
£Ø4£©ŅŅ“¼·Ö×ÓÖŠµÄŃõŌ×Ó±»ĮņŌ×ÓČ”“śŗóµÄÓŠ»śĪļ½ŠŅŅĮņĖį£¬ĘäŠŌÖŹÓėŅŅ“¼ĻąĖĘ£¬µ«ŅŅ“¼ÓŠĖįŠŌ£¬ĖüÄÜÓėNaOHµČĒæ¼īČÜŅŗ·“Ӧɜ³ÉĪŽÉ«ČÜŅŗ”£ĘūÓĶÖŠŅņŗ¬ŅŅĮņ“¼£ØĪŽÉ«ŅŗĢ壬Ī¢ČÜÓŚĖ®£©Ź¹ĘūÓĶÓŠ³ōĪ¶ĒŅĘäČ¼ÉÕ²śÉśµÄ¶žŃõ»ÆĮņ»įĪŪČ¾»·¾³£¬Ņņ“ĖŅŖ°ŃĖü³żČ„”£
¢ŁÓĆ»Æѧ·½·Ø³żČ„ĘūÓĶѳʷ֊µÄŅŅĮņ“¼£¬ĒėŠ“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
¢ŚŅŅĮņ“¼ÓėŅŅ“¼ŌŚŅ»¶ØĢõ¼žĻĀæÉ·¢Éśõ„»Æ·“Ó¦£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
¢ŪŌŚ120”ę”¢1.01”Į105PaŹ±£¬½«ŅŅ“¼”¢ŅŅĮņ“¼µÄ»ģŗĻÕōĘų1LŗĶ5LO2»ģŗĻ£¬³ä·ÖČ¼ÉÕŗ󣬻Öø“µ½Ōדæö£¬ĘųĢåĆÜ¶Č¼õŠ”ĪŖČ¼ÉÕĒ°»ģŗĻĘųĢåµÄ10/11£¬ŌņŅŅ“¼ŗĶŅŅĮņ“¼µÄĢå»żÖ®±ČĪŖ ”£
”¾½āĪö”æøĆĢāæ¼²éĮĖÓŠ»śĪļµÄ»ł±¾ÖŖŹ¶£¬Ķ¬Ź±»¹æ¼²éĮĖѧɜ¶ŌÖŖŹ¶µÄĒØŅĘÄÜĮ¦”£
£Ø1£©ĪŹŹĒ¶Ō»ł“”ÖŖŹ¶µÄ漲飬±Č½Ļ¼ņµ„”£
£Ø2£©Ē°Į½ÖÖ·½·ØÓƵ½ŅŅĻ©ŗĶäåŅŅĶ飬ĖüĆĒ¶¼ŹĒŅŌŹÆÓĶĪŖŌĮĻÖĘµĆ²śĘ·£¬ŹÆÓĶŹĒ²»æÉŌŁÉś×ŹŌ“”£¶ųÓƵķ·ŪĪŖŌĮĻ£¬µķ·ŪŹĒæÉŌŁÉś×ŹŌ“£¬ĖłŅŌŃ”ÓƵŚČżÖÖ·½·Ø”£
£Ø3£©Ō×ÓĄūÓĆĀŹ“óµÄŹĒµŚŅ»ÖÖ·½·Ø£¬Ö»ÓŠŅŅ“¼Éś³É£¬Ć»ÓŠĘäĖūĪļÖŹÉś³É”£
£Ø4£©×„×”ŅŅĮņ“¼ŠŌÖŹÓėŅŅ“¼ĻąĖĘÕāŅ»µć£¬»įŠ“ŅŅ“¼ÓėĒāŃõ»ÆÄʵķ“Ó¦¾Ķ»įŠ“ŅŅĮņ“¼µÄ·“Ó¦£¬Ķ¬ĄķæÉŠ“³öŅŅĮņ“¼ÓėŅŅĖįµÄ·“Ó¦”£¢ŪĘųĢåĆÜ¶Č¼õŠ”ĪŖČ¼ÉÕĒ°»ģŗĻĘųĢåµÄ![]() £¬ŌņĘųĢåĢå»żŌö“óĪŖŌĄ“µÄ
£¬ŌņĘųĢåĢå»żŌö“óĪŖŌĄ“µÄ![]() £¬·¢ÉśĮ½øö·“Ó¦CH3CH2OH+3O2”ś2CO3+2H2O¢Ł£¬CH3CH2SH+
£¬·¢ÉśĮ½øö·“Ó¦CH3CH2OH+3O2”ś2CO3+2H2O¢Ł£¬CH3CH2SH+![]() O2”ś2CO2+SO2+3H2O¢Ś£¬”÷V1=1L”£”÷V2=0.5L£¬ÉčŌŚ120”ę1.01”Į105PaŹ±£¬ŅŅ“¼µÄĢå»żĪŖX£¬ŅŅĮņ“¼Ģå»żĪŖY£¬ŌņX+Y=1L£¬X+0.5Y=6.6L£6L£»½āµĆX=0.2L,Y=0.8L£»ĖłŅŌĢå»ż±ČĪŖ1£ŗ4”£
O2”ś2CO2+SO2+3H2O¢Ś£¬”÷V1=1L”£”÷V2=0.5L£¬ÉčŌŚ120”ę1.01”Į105PaŹ±£¬ŅŅ“¼µÄĢå»żĪŖX£¬ŅŅĮņ“¼Ģå»żĪŖY£¬ŌņX+Y=1L£¬X+0.5Y=6.6L£6L£»½āµĆX=0.2L,Y=0.8L£»ĖłŅŌĢå»ż±ČĪŖ1£ŗ4”£
“š°ø£ŗ£Ø1£©CH2=CH2+H2O ![]() CH3CH2OH Č”“ś·“Ó¦
CH3CH2OH Č”“ś·“Ó¦
£Ø2£©Ó¦øĆÓĆ·½·Ø¢Ū£¬ŅņĪŖµķ·ŪŹĒæÉŌŁÉś×ŹŌ“£¬¶ųŅŅĻ©”¢äåŅŅĶ鶼ŹĒŅŌŹÆÓĶĪŖŌĮĻÖĘµĆµÄ²śĘ·£¬ŹÆÓĶŹĒ²»æÉŌŁÉś×ŹŌ“”£
£Ø3£©A
£Ø4£©¢ŁCH3CH2OH+NaOH”śCH3CH2SNa+H2O
¢ŚCH3COOH+CH3CH2SH ![]()
![]()
¢Ū1£ŗ4


 ³¤½×÷Ņµ±¾Ķ¬²½Į·Ļ°²įĻµĮŠ“š°ø
³¤½×÷Ņµ±¾Ķ¬²½Į·Ļ°²įĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ”÷ |
| µķ·ŪĆø |
| ¾Ę»ÆĆø |
| “߻ƼĮ |
| “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹŠÄāĶ¶×Ź½ØÉčŅ»øö¹¤Ņµ¾Ę¾«³§£¬ÄæµÄŹĒÓĆ¹¤Ņµ¾Ę¾«ÓėĘūÓĶ»ģŗĻÖĘ³É”°ŅŅ“¼ĘūÓĶ”±£¬ŅŌ½ŚŹ”ŹÆÓĶ׏Ō“£¬¼õÉŁ“óĘųĪŪČ¾”£ŅŃÖŖÖĘ¾ĘµÄ·½·ØÓŠČżÖÖ£ŗ
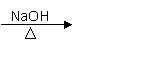 ¢ŁŌŚ“߻ƼĮ×÷ÓĆĻĀŅŅĻ©ÓėĖ®·“Ó¦
¢ŁŌŚ“߻ƼĮ×÷ÓĆĻĀŅŅĻ©ÓėĖ®·“Ó¦
¢ŚCH3CH2Br+H2O CH3CH2OH+HBr
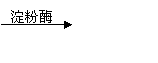 ¢Ū£ØC6H10O5£©n£Øµķ·Ū£©+nH2O nC6H12O6£ØĘĻĢŃĢĒ£©
¢Ū£ØC6H10O5£©n£Øµķ·Ū£©+nH2O nC6H12O6£ØĘĻĢŃĢĒ£©
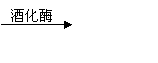
C6H10O6£ØĘĻĢŃĢĒ£© 2C2H5OH+2CO2”ü
£Ø1£©·½·Ø¢Ł·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£·“Ó¦ĄąŠĶĪŖ £¬·½·Ø¢ŚµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ”£
£Ø2£©ĪŖ»ŗ½āŹÆÓĶ¶Ģȱ“ųĄ“µÄÄÜŌ“Ī£»ś£¬ÄćČĻĪŖøĆŹŠÓ¦Ń”ÓĆÄÄŅ»ÖÖ·½·ØÉś²ś¹¤Ņµ¾Ę¾«£æĒė¼ņŹöĄķÓÉ ”£
£Ø3£©Čē¹ū“ÓĀĢÉ«»Æѧ£Ø”°Ō×ÓĄūÓĆĀŹ”±×ī“ó»Æ£©µÄ½Ē¶Čæ“£¬ÖĘ¾Ę¾«×īŗƵÄŅ»×é·½·ØŹĒ
£ØĢīŠņŗÅ£©
A£®¢Ł B£®¢Ū C£®¢Ł¢Ū D£®¢Ł¢Ś¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”Ī«·»ŹŠÖŲµć֊ѧŠĀø߶žŹī¼Ł»Æѧ×÷Ņµ£ØĪ壩£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
 CH3CH2OH+HBr
CH3CH2OH+HBr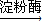 nC6H12O6£ØĘĻĢŃĢĒ£©
nC6H12O6£ØĘĻĢŃĢĒ£©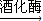 2C2H5OH+2CO2”ü
2C2H5OH+2CO2”ü²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹŠÄāĶ¶×Ź½ØÉčŅ»øö¹¤Ņµ¾Ę¾«³§£¬ÄæµÄŹĒÓĆ¹¤Ņµ¾Ę¾«ÓėĘūÓĶ»ģŗĻÖĘ³É”°ŅŅ“¼ĘūÓĶ”±£¬ŅŌ½ŚŹ”ŹÆÓĶ׏Ō“”£ŅŃÖŖÖĘ¾Ę¾«µÄ·½·ØÓŠČżÖÖ£ŗ¢ŁŌŚ“߻ƼĮ×÷ÓĆĻĀŅŅĻ©ÓėĖ®·“Ó¦ ¢ŚCH3CH2Br+H2O![]() CH3CH2OH+HBr ¢Ū(C6H10O5)n(µķ·Ū)+nH2O
CH3CH2OH+HBr ¢Ū(C6H10O5)n(µķ·Ū)+nH2O![]() nC6H12O6(ĘĻĢŃĢĒ)
nC6H12O6(ĘĻĢŃĢĒ)
C6H12O6(ĘĻĢŃĢĒ)![]()
(1)·½·Ø¢ŁµÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ_________________________________________________”£
(2)·½·Ø¢ŚµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ_______________”£
(3)Čē¹ū“ÓĀĢÉ«»Æѧ(”°Ō×ÓĄūÓĆĀŹ”±×ī“ó»Æ)µÄ½Ē¶Čæ“£¬ÖĘ¾Ę¾«×īŗƵÄŅ»×é·½·ØŹĒ_______________”£
A.¢Ł B.¢Ū C.¢Ł¢Ū D.¢Ł¢Ś¢Ū
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com