
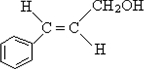

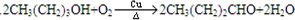
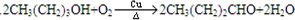
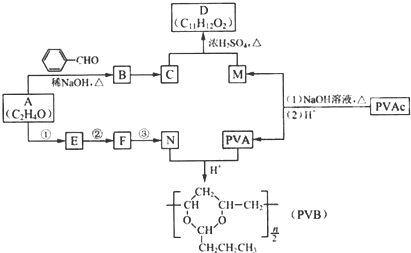
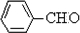 �����ӳɷ�Ӧ��IJ��BΪ
�����ӳɷ�Ӧ��IJ��BΪ 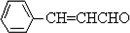 ����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ
����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ ������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ
������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ 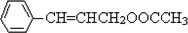 ������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ
������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ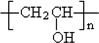 �������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪCH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮
�������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪCH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮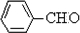 �����ӳɷ�Ӧ��IJ��BΪ
�����ӳɷ�Ӧ��IJ��BΪ 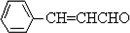 ����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ
����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ ������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ
������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ 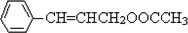 ������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ
������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ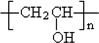 �������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪCH3CH=CHCHO��FΪCH3��CH2��3OH��
�������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪCH3CH=CHCHO��FΪCH3��CH2��3OH��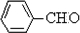 �����ӳɷ�Ӧ��IJ������B�Ľṹ��ʽΪ
�����ӳɷ�Ӧ��IJ������B�Ľṹ��ʽΪ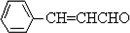 ���ʴ�Ϊ��
���ʴ�Ϊ��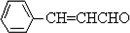 ��
�� ��
�� ��
�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
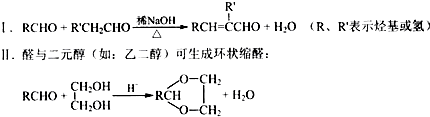
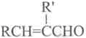 +H2O��R��R����ʾ�������⣩
+H2O��R��R����ʾ�������⣩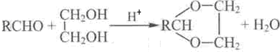
 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��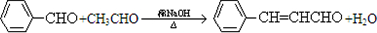
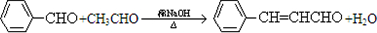


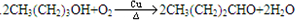
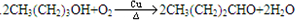
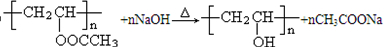
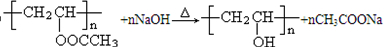
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
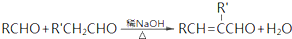 ��R��R�@��ʾ�������⣩
��R��R�@��ʾ�������⣩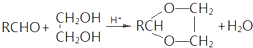
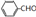 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��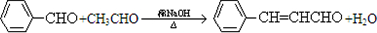 ��
��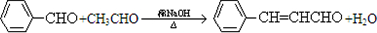 ��
��

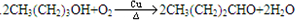
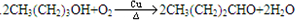
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
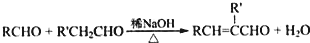 ��R��R���ʾ������
��R��R���ʾ������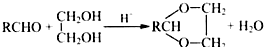


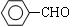 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��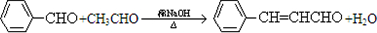
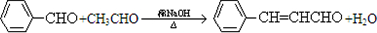
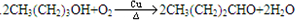
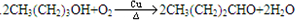
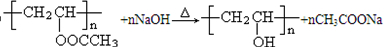
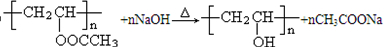
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ���������� ���ͣ������
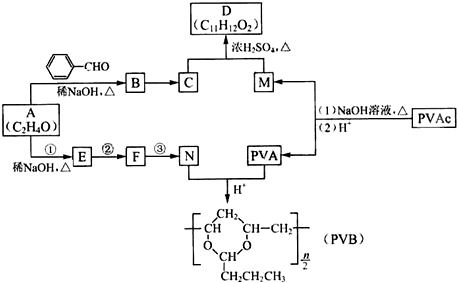
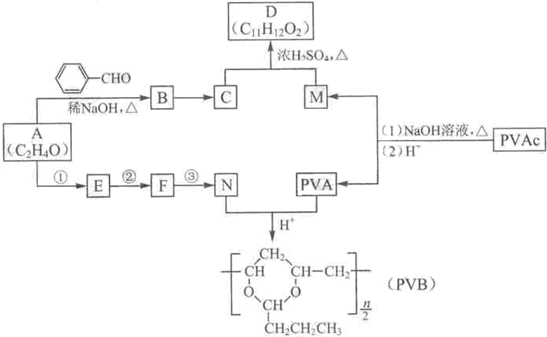
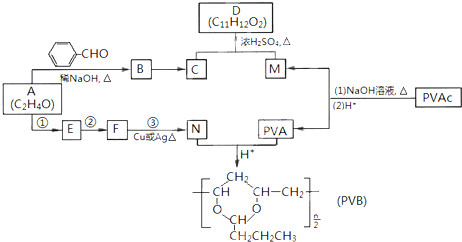
�����������ӵ��㾫�Ķ����D�Լ���������ȫ�����в�ĸ߷��ӻ�����PVB�ĺϳ�·�����£�
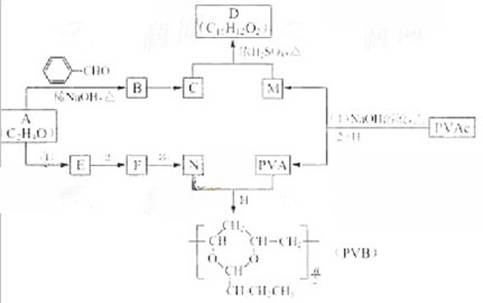
��֪��
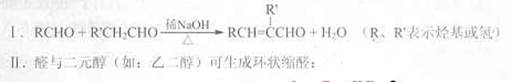
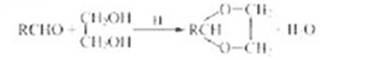
��1��A�ĺ˴Ź������������ַ壬A��������
��2��A��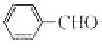 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��
��3��CΪ��ʽ�ṹ����B��ԭ�õ���C�Ľṹ����
��4��E��ʹ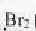 ��
�� ��Һ��ɫ��N��A����Ӧ1~3�ϳɡ�
��Һ��ɫ��N��A����Ӧ1~3�ϳɡ�
a. �ٵĻ�ѧ�Լ��������� ��
b. �ڵķ�Ӧ������ ��
c. �۵Ļ�ѧ����ʽ�� ��
��5��PVAC��һ�ֵ��徭�Ӿ۷�Ӧ�õ����õ���Ľ����ʽ�� ��
��6�����������£�PVAc��ȫˮ��Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com