
����Ŀ���Ա�����ѧ��������Ϊ�����Ķ���Ŷӣ��з����˸�Ч������(��FeC��Fe2C��Fe5C2��Fe3C)���кϳɴ�������H2��COΪԭ�Ͽɸ߲��ʺϳ�ϩ������������3CO(g)+ 6H2(g)![]() CH3CH=CH2(g)+3H2O(g)��nCO+(2n+1)H2
CH3CH=CH2(g)+3H2O(g)��nCO+(2n+1)H2![]() CnH2n+2+nH2O��Ϊú��������Һ��ʹ�ÿ�������;����
CnH2n+2+nH2O��Ϊú��������Һ��ʹ�ÿ�������;����
(1)Fe3+��������ߵ��ܼ��ϵĵ�������_____����չ����λ�ڲ�ͬ�������˶��ĵ��ӵ�������С��ϵ��_________����Feԭ�ӵ����Ų���[Ar]3d64s2��[Ar]3d64s14p1ʱ����ϵ������_______(����������������С��)��
(2)Fe��C��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_____________��CH3CH=CH2������̼ԭ�ӵ��ӻ��������Ϊ____�����������Ӧ��CO�����ж��ѵĻ�ѧ������Ϊ______(����ĸ)��
A. 2��������1������ B. 1��������2������ C. �Ǽ��Լ�
(3)������[(CH3)4C]������5��̼ԭ���γɵĿռ乹����____________���÷�����________(���������������Ǽ�����)���ӡ�������������̼ԭ����Ŀ�����ӣ�ͬϵ��ķе�����.��ԭ����__________��
(4)̼��֮����γɶ��ֻ��������һ�ֻ�����ľ���ṹ(���������ṹ)��ͼ��ʾ��

����Ϊ�ٵ�ԭ�ӵ�����Ϊ_______________���û�����Ļ�ѧʽΪ_______________����þ���ľ�������Ϊa pm�������ӵ�������ֵΪNA����þ�����ܶ�Ϊ_______________ g��cm-3(�г�����ʽ����)��
���𰸡�5 ��� ���� O>C>Fe sp2��sp3 B �������� �Ǽ��� ����̼ԭ����Ŀ���ӣ����Ӽ����������� (0��0��![]() ) FeC
) FeC 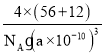
��������
(1)�ȸ��ݹ���ԭ����дFeԭ�Ӽ�Fe3+�ĺ�������Ų���Ȼ���ϵ�������������������״�����жϣ�ͬһ����ϵ���������ͬ����ͬ����ϵ��ӵ�������ͬ��ԭ�����������������ɻ�̬��Ϊ����̬��
(2)Ԫ�صķǽ�����Խǿ��������ܾ�Խ����̼ԭ�Ӳ���sp3�ӻ�������̼̼˫����̼ԭ�Ӳ���sp2�ӻ���CO����������ԭ�ӹ������Ե��ӣ�
(3)����̼ԭ���γɵ���������ṹ�����γɵĹ��ۼ�������ͬ�������������壻���ӽṹ�ԳƵľ��ǷǼ��Է��ӣ�����Ϊ���Է��ӣ�����֮��ͨ�����Ӽ���������ϣ����Ӽ�������Խ�����ʵ��۷е��Խ�ߣ�
(4)���ݢ�������ϵ���������ԭ���λ�ã��ж�������ֵ������̼��������ľ���ṹ���þ�̯���������仯ѧʽ��������=![]() �����ܶȡ�
�����ܶȡ�
(1)26��Feԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d64s2��Feԭ��ʧȥ2��4s���Ӻ�1��3d���ӵõ�Fe3+��������Ų�ʽ��1s22s22p63s23p63d5���ɼ�Fe3+��������ߵ��ܼ���3d����������5�ֲ�ͬ����չ����3d�ܼ��ϵ��ӵ�������ȡ���Feԭ�ӵ����Ų���[Ar]3d64s2��[Ar]3d64s14p1ʱ��ԭ���ɻ�̬��ɼ���̬ʱ��Ҫ��������������ϵ��������
(2)����Ԫ�صĵ�һ�����ܱȷǽ���Ԫ�ص�С����Ԫ�صĵ�һ�����ܴ���̼Ԫ�صĵ�һ�����ܡ�������Ԫ�صĵ�һ�������ɴ�С��˳��ΪO>C>Fe����ϡ��������һ����̼ԭ���DZ���̼ԭ�ӣ��γ���4������������sp3�ӻ�����������̼ԭ���γ���̼̼˫����ÿ��̼ԭ���γ���3��������1��������Ϊsp2�ӻ���CO��N2��Ϊ�ȵ����壬�ɴ˿�֪CO��������2��������1����������ͬԪ�ص�ԭ�Ӽ��γɵĹ��۽��Ǽ��Թ��ۼ����ʺ���ѡ����B��
(3)�������������4�������ӵ�Cԭ����4�����ϵ�Cԭ���γ���һ����������ṹ�����ڽṹ�Գƣ�������ǷǼ��Է��ӡ�������ͬϵ�����ɡ��ṹ���ƣ���Ϊ���Ӿ��壬���Ӽ����������ŷ�����̼ԭ����Ŀ�����Ӷ��������������������̼ԭ����Ŀ�����ӣ�ͬϵ��ķе����ߣ�
(4)�۲쾧��ͼ֪���ٴ�̼ԭ�ӵ�x=y=0��z=+![]() ���ʢ�����Ϊ(0��0��
���ʢ�����Ϊ(0��0��![]() )���ɾ�̯������֪����һ�������к���C��12��
)���ɾ�̯������֪����һ�������к���C��12��![]() +1=4������Feԭ�ӣ�8��
+1=4������Feԭ�ӣ�8��![]() +6��
+6��![]() =4�����仯ѧʽΪFeC������������Ϊ4��(56+12) g���������Ϊ(a��10-10 cm)3���ʾ����ܶ���=
=4�����仯ѧʽΪFeC������������Ϊ4��(56+12) g���������Ϊ(a��10-10 cm)3���ʾ����ܶ���=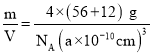 =
=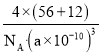 g/cm3��
g/cm3��


 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������µķ�Ӧ��3H2 (g)+N2 (g) ![]() NH3 (g)���ﵽ�ȵı�־��( )
NH3 (g)���ﵽ�ȵı�־��( )
A.H2��N2��NH3�ķ��Ӹ���֮��Ϊ3��1��2B.N2������Ӧ���ʺ��淴Ӧ�������
C.��Ӧ�Ѿ�ֹͣD.��λʱ��������3mol H2��ͬʱ����1mol N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����0.55mol/LNaOH��Һ��0.50mol/LHCl��Һ�����к��ȵIJⶨ���ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ��

(1)��ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ�������__________��
(2)ʹ�ò�ȫ�������װ�ý���ʵ�飬ȡ50mL0.50mol/LHCl��Һ��50mL0.55mol/LNaOH��Һ��С�ձ��н����кͷ�Ӧ��ʵ�����������
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ(t2-t1)/�� | ||
HCl | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ______�� |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
������д���еĿհף��¶Ȳ�ƽ��ֵ__________��
������ʵ����ֵ������к���57.3kJ/molƫС��������ԭ�������______��(����ĸ)
a.ʵ��װ�ñ��¡�����Ч����
b.��ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶�
(3)ʵ��������60mL0.25mol/LH2SO4��Һ��50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������______(���ȡ���������ȡ�)�������к���______(���ȡ���������ȡ�)������50mL0.50mol/L�������HCl��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ��______(�ƫ����ƫС����������Ӱ�족)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C2H4)�ǻ������л�����ԭ�ϣ���A�Ʊ��۱�ϩ��������л�������Ҫ�ɷ֣��������ĺϳ�·�ߣ����ַ�Ӧ������ȥ������ͼ��ʾ��
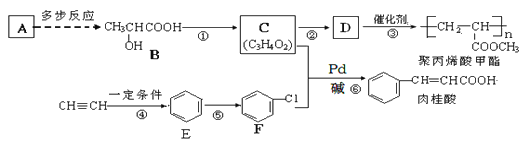
��֪��![]() +CH2=CH-R
+CH2=CH-R![]() +HX��XΪ±ԭ�ӣ�RΪȡ������
+HX��XΪ±ԭ�ӣ�RΪȡ������
�ش��������⣺
��1����Ӧ�ٵķ�Ӧ������_______���ķ�Ӧ������_____��
��2��B������Ϊ_______________________________��
��3����C��ȡD�Ļ�ѧ����ʽΪ__________��
��4��������ͬ���칹������ͬʱ���������������ٱ�����������ȡ�����ұ����ϵ�һ�ȴ��������֢��ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ����д����������Ҫ�����ʵĽṹ��ʽ��________��
��5����֪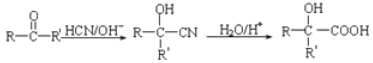 ��A�Ʊ�B�ĺϳ�·�����£����в��ַ�Ӧ������ʡ�ԣ���
��A�Ʊ�B�ĺϳ�·�����£����в��ַ�Ӧ������ʡ�ԣ���
CH2=CH2��X ![]() Y��Z
Y��Z![]()
![]()
��X��Y��Ӧ����ʽΪ______________�� Z�Ľṹ��ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Sb)�ǵ�VA��Ԫ�أ��䵥����Ҫ��������Ͻ𡢰뵼�塣�����������׳���ף��ǰ�ɫ��ĩ��������ˮ����һ�������������Ҫ���ڰ�ɫ���ϡ���������ϡ�ʯ�ͻ����ȡ�ij��������ë��(��Ҫ�ɷ�ΪPb4FeSb6S14)��ȡ��Ĺ���������ͼ��ʾ��

(1)Pb4FeSb6S14�е���Ԫ��ֻ��һ�ֻ��ϼۣ����仯�ϼ���______��X��һ�ֹ��嵥�ʣ���ɷ���___(�ѧʽ)��
(2)�Ȼ������У���Ǧ��X�⣬��������Ԫ�ط�Ӧ����Ըۣ�д����Ӧ�Ļ�ѧ����ʽ��______________������1Ϊ��ˮϡ�ͣ�д������SbOCl�����ӷ���ʽ��___________��
(3)�Լ�1ͨ��ѡ�ð�ˮ������NaOH��Һ������ܵ�ԭ����_______������2��������_________�����
(4)��ǿ���������µ��Na3SbS3��Һ(ԭ����ͼ)�ɵõ������ࡣ

д�������ĵ缫��Ӧʽ��_______��B�缫Ӧ�ӵ�Դ��______��������2 mol Sb����ʱ��ͨ�������ӽ���Ĥ��������Ϊ_________(��NAΪ�����ӵ�������ֵ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() Ϊ�����ӵ�������ֵ�����������������( )
Ϊ�����ӵ�������ֵ�����������������( )
A.![]()
![]() ��������Һ���е�
��������Һ���е�![]() ��ĿΪ
��ĿΪ![]()
B.![]()
![]() ��
��![]() �Ļ�������к���
�Ļ�������к���![]() ������ĿΪ
������ĿΪ![]()
C.7g��ϩ�ͻ�����![]() �Ļ�������к��е���ԭ����ĿΪ
�Ļ�������к��е���ԭ����ĿΪ![]()
D.![]()
![]() ��Ũ
��Ũ![]() �����
�����![]() ��Ӧ��ת�Ƶĵ���������
��Ӧ��ת�Ƶĵ���������![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ֱ�Ϊ�������ڷ��ӵIJ��ֽṹģʽͼ�����ͼ�ش����⣺
��1����ͼ�е��������ʷֱ����ڶ��ֲ��ϸ���еĴ������ʵ���_____��������������ʵĵ��嶼��_____��
��2����ͼ��ʾ������Ļ�����ɵ�λ��___________����ͼ����ĸ_______________ ��ʾ�Ľṹ��ʾ����������λ֮����ͨ��_____________(��١����ڡ��ۡ�)�������� �ġ�
��3����ͼ����________�������ᾭ__________ �����γɵģ�������ĽṹͨʽΪ__________ ����������������______________ �Լ���
��4���������е� Mg2+��Fe2+���������ξ���ʲô���ܣ�_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2020��3��9�գ��ҹ����������Ƿ��������ó������������ػ�����ɹ����䱱��ϵͳ��54�ŵ������ǡ��������������ػ��һ���Ӽ�ʹ��Һ��������������Һ��ƫ������(![]() ���ֳ�1��1-��������)��Ϊ�ƽ����������Ӽ�ʹ��Һ���Һ����Ϊ�ƽ����������ƽ���ȼ�յIJ����Ϊ�����ʡ�����˵����ȷ����( )
���ֳ�1��1-��������)��Ϊ�ƽ����������Ӽ�ʹ��Һ���Һ����Ϊ�ƽ����������ƽ���ȼ�յIJ����Ϊ�����ʡ�����˵����ȷ����( )
A.ƫ�����·����м��м��Լ�Ҳ�зǼ��Լ������ڷǼ��Է���
B.ȼ��ʱÿ����![]() ƫ�����»�ת��
ƫ�����»�ת��![]() ����
����
C.��![]() Һ��������Һ����ȫ��Ӧ����Һ̬ˮ���ͷ�
Һ��������Һ����ȫ��Ӧ����Һ̬ˮ���ͷ�![]() ��������������ȼ����Ϊ
��������������ȼ����Ϊ![]()
D.�����ƽ���ȼ�յIJ��ﲻ������κλ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������H2��ԭWO3�Ʊ�����W��װ����ͼ��ʾ��Zn������������������ʣ�����ûʳ������Һ������������������������˵����ȷ����
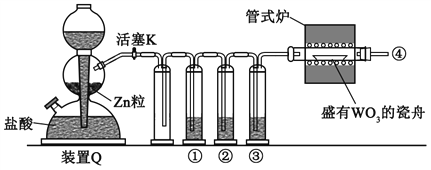
A. �١��ڡ���������ʢװKMnO4��Һ��ŨH2SO4������ûʳ������Һ
B. ��ʽ¯����ǰ�����Թ��ڢܴ��ռ����岢��ȼ��ͨ�������ж����崿��
C. ������Ӧʱ���ȹرջ���K����ֹͣ����
D. װ��Q�����շ�������Ҳ�����ڶ���������Ũ���ᷴӦ�Ʊ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com