
 ��ѧ��ȤС���趨����ʵ�鷽�����ⶨij�ѱ���Ϊ̼���Ƶ�С�մ���Ʒ��NaHCO3������������
��ѧ��ȤС���趨����ʵ�鷽�����ⶨij�ѱ���Ϊ̼���Ƶ�С�մ���Ʒ��NaHCO3���������������� [����һ]̼�����Ʋ��ȶ��������ֽ⣬���ݼ���ǰ����������仯�����ݲ�������̼�����Ƶ��������������̼���Ƶ�����������
��1��̼���������ȷֽ�����̼���ơ�ˮ�Ͷ�����̼��
��2�����Ⱥ��ر�֤̼��������ȫ�ֽ⣻
[������]̼���ƺ�̼�����ƶ�������������Ӧ����̼�ᱵ�������������ɳ���������������̼���Ƶ�����������
��1�����ݹ��˲�����������������
��2����ȡ�ϲ���Һ�������ӳ����������Ƿ����ɳ�����
[������]��3���ɲⶨ������ʵ���֪��A�з���Na2CO3+H2SO4=H2O+CO2��+Na2SO4��2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2����B��ΪŨ��������ˮ�����������̼������Cװ�����ն�����̼��Dװ�÷�ֹ�����еĶ�����̼��ˮ����Cװ�ø��ź����ⶨ��
��4����Ϸ�Ӧ������ϵ��Ԫ���غ����õ����ʵ����������������ʺ�����
��5��������̼���岻��ȫ������Cװ�ñ����գ���Ҫ����һ��װ�ö�����̼�������װ��C��װ�ã�
��� �⣺[����һ]��1��̼���������ȷֽ�����̼���ơ�ˮ�Ͷ�����̼����Ӧ����ʽΪ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
�ʴ�Ϊ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
��2��ʵ��ԭ���Ǹ��ݼ���ǰ����������仯������̼�����ƣ���Ӧ��֤̼��������ȫ�ֽ⣬���Ⱥ�����̼��������ȫ�ֽ⣬
�ʴ�Ϊ����֤NaHCO3�ֽ���ȫ��
[������]��1������ʱ���ò������������ʴ�Ϊ����������
��2����ȡ�ϲ���Һ�������ӳ����������Ƿ����ɳ������������Ϊ��ȡ�ϲ���Һ�������Թ��У���������������Һ�����������ɣ��������ȫ��
�ʴ�Ϊ��ȡ�����ϲ���Һ��һ֧�Թ��У��μ�Ba��OH��2��Һ���۲��Ƿ��а�ɫ�������ɣ�
[������]��1��B��ΪŨ��������ˮ�����������̼�������е�ˮ�����Ͷ�����̼�ᱻ��ʯ�����գ���D�����������տ����е�ˮ�����Ͷ�����̼����ȷ��Cװ����������������ȷ�ԣ���Һ©�������������������ᣬ�����ӷ��������Ƶö�����̼�����к��Ȼ��⣬Ũ����������Ȼ��⣬���Ȼ��ⱻ��ʯ�����գ����²������̼����ƫ�ߣ�������̼���ƺ�̼�����ƣ�̼�����Ʋ���������̼�࣬��ᵼ��̼������ƫ�̼࣬����ƫС��
�ʴ�Ϊ��Ũ�����ֹ�����е�ˮ������������̼����Cװ�ã����ܣ�
��2����Na2CO3��NaHCO3�����ʵ����ֱ�Ϊx��y����
Na2CO3+H2SO4=H2O+CO2��+Na2SO4��
1 1
x x
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2 2
y y
106x+84y=17.90
44x+44y=8.80
���x=0.05mol
y=0.15mol
����Ʒ��NaHCO3����������Ϊ=$\frac{0.15mol��84g/mol}{17.9g}$��100%=70.4%��
�ʴ�Ϊ��70.4%��
��3��ʵ��װ�û�����һ������ȱ��Ϊװ���еĶ�����̼���ܱ�Cȫ�����գ��������һ��װ�ý�A��B�е�CO2ȫ������C�����գ�
�ʴ�Ϊ��ȱ��һ��A��Bװ���ڵ�CO2�������ϵ�Cװ���е�װ�ã�
���� ���⿼��̼���ƺ����IJⶨʵ�飬Ϊ��Ƶ���㣬����ʵ��װ�õ����ü�ʵ��Ŀ��Ϊ���Ĺؼ������ط��������㼰ʵ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4L SO3�к��еķ�����ΪNA�� | |
| B�� | 2L 0.5 mol•L-1��������Һ�к��е�H+������Ϊ2NA | |
| C�� | ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.2NA | |
| D�� | �ܱ�������2mol NO��1mol O2��ַ�Ӧ������ķ�����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���������� | B�� |  ������������ | C�� | 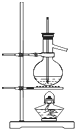 ʯ�͵����� | D�� |  ʵ��������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molH3PO4������Ϊ98g•mol-1 | B�� | H3PO4��Ħ������Ϊ98g | ||
| C�� | 9.8g H3PO4����NA��H3PO4���� | D�� | NA��H3PO4���ӵ�����Ϊ98g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ż�������ˮ���� | |
| B�� | ϡ��Ũ����ʱ����Ũ��������������ע��ˮ�� | |
| C�� | Ƥ���ϲ���մ��NaOH��Һ�������������ϴ | |
| D�� | ʵ����������촵��ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ij�����о�С���ͬѧ�ڲ�������ʱ��֪��Na2O2������CO2���ܷ�����Ӧ����������ˮ����ʱ��Na2O2����CO2������Ӧ����Na2CO3��O2��Ϊ��̽����������̼�Ƿ�����ˮ����ʱ������������Ʒ�Ӧ����ij�����о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺
ij�����о�С���ͬѧ�ڲ�������ʱ��֪��Na2O2������CO2���ܷ�����Ӧ����������ˮ����ʱ��Na2O2����CO2������Ӧ����Na2CO3��O2��Ϊ��̽����������̼�Ƿ�����ˮ����ʱ������������Ʒ�Ӧ����ij�����о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ����ʵ��Ϊ��������Ȼ��ѧ | |
| B�� | ��ѧ��һ�Ŵ����Եġ�ʵ���ԵĿ�ѧ | |
| C�� | ����Ļ�ѧʵ�����ʼ�ڽ��� | |
| D�� | �ִ���ѧ���ɳ�֮Ϊ21���͵����Ŀ�ѧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com