
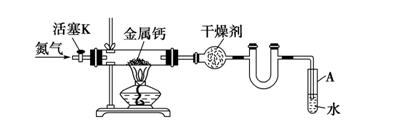
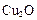 ����Ϊ̫����ֽ�ˮ�Ĵ�����
����Ϊ̫����ֽ�ˮ�Ĵ�����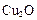 �ķ���
�ķ���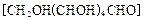 ��ԭ���Ƶ�
��ԭ���Ƶ�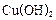 ��д����ѧ����ʽ ��
��д����ѧ����ʽ ��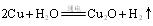 �������������� ��
�������������� ��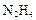 ����ԭ����
����ԭ����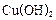 ���Ʊ�����
���Ʊ�����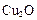 ��ͬʱ�ų�
��ͬʱ�ų�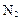 �����Ʒ��Ļ�ѧ����ʽΪ ��
�����Ʒ��Ļ�ѧ����ʽΪ ��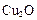 ���д��ֽ�ˮ��ʵ��
���д��ֽ�ˮ��ʵ��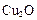 ��ͨ��0��10molˮ������������Ӧ��
��ͨ��0��10molˮ������������Ӧ��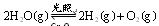 ����H= +484kJ/mol����ͬʱ�β���
����H= +484kJ/mol����ͬʱ�β���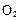 �������±���
�������±���| ʱ��/min | 20 | 40 | 60 | 80 |
| n��O2��/mol | 0��0010 | 0��0016 | 0��0020 | 0��0020 |
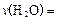 ����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��
����ƽ��ʱ��������Ҫ���յĹ���Ϊ kJ��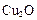 ��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��
��ij��ͬ�����·ֱ��ˮ���ֽ⣬��������������v��ʱ��t�仯��ͼ��ʾ������������ȷ���� ��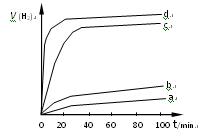
A��c��d�����Ƶõ�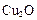 ��Ч����Խϸ� ��Ч����Խϸ� |
B��d�����Ƶõ�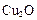 ������ʱ��ˮ��ƽ��ת������� ������ʱ��ˮ��ƽ��ת������� |
C����Ч����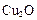 �����Ĵ�ϸ��������Ե��й� �����Ĵ�ϸ��������Ե��й� |
| D��Cu2O��ˮ�ֽ�ʱ����Ҫ���˵��¶� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
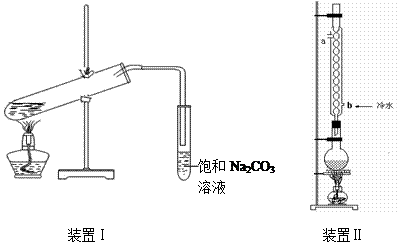
| �� �� |
| �е�/�� | �ܶ�/g��cm��3 | ||
| �� �� | ��114 | 78 | 0.789 | ||
| �� �� | 16.6 | 117.9 | 1.05 | ||
| �������� | ��83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
 ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ��������� �������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
�������ã� ��������Ȼ�����_____�ڣ���ϡ����¡����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
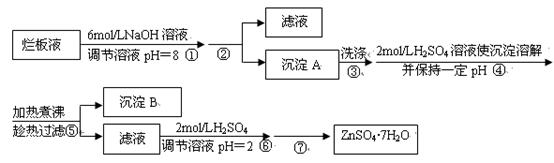
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Zn2+ | 6.4 | 8.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO | B��NH3 | C��C2H4 | D��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������֬���ռ��Ʒ�����������ˮ�ⷴӦ |
| B���õ�ʯ��ˮ��Ӧ����Ȳ��������ԭ��Ӧ |
| C����NH4Cl����ʯ�ҷ�Ӧ�ư����Ǹ��ֽⷴӦ |
| D���ñ���FeCl3��Һ��Fe��OH��3������ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
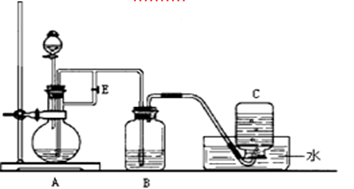
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 Ӧ�õ���b���ڿɼ�������������b��ĩ�˸���ƿ��Һ�汣��һ�ξ����Ŀ����___________________��
Ӧ�õ���b���ڿɼ�������������b��ĩ�˸���ƿ��Һ�汣��һ�ξ����Ŀ����___________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
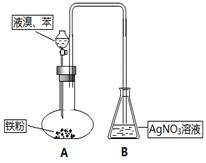
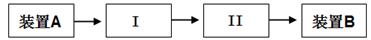
| A��װ��NaOH��Һ��ϴ��ƿ | B��װ��CC14��ϴ��ƿ |
| C��װ��KI��Һ��ϴ��ƿ | D��װ��ʪ�����KI��ֽ�ļ���ƿ |
 ���ϲ����У���֤������ϴ�Ӹɾ��ķ�����_______________________________________��
���ϲ����У���֤������ϴ�Ӹɾ��ķ�����_______________________________________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com