
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���________���ڵ�________�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ________________________��
(2)�á�>����<����գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si____S | O2��____Na�� | NaCl____Si | H2SO4____HClO4 |
(3)CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܴﵽ����(����)
A����58.5 g NaCl����1 Lˮ�пɵ�1 mol/L��NaCl��Һ
B������״����22.4 L HCl����1 Lˮ�пɵ�1 mol/L����
C ����25.0 g��������ˮ�����100 mL��Һ������ҺŨ��Ϊ1 mol/L
����25.0 g��������ˮ�����100 mL��Һ������ҺŨ��Ϊ1 mol/L
D����78 g Na2O2����ˮ�����1 L��Һ�ɵõ�Ũ��Ϊ2 mol/L��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж������йػ�ѧ���������������ȷ����(����)
A�����壺���ʵ�����ֱ���Ƿ���1 nm��100 nm֮��
B��������ԭ��Ӧ����Ӧǰ��Ԫ�صĻ��ϼ��Ƿ�仯
C�����ۻ�������ɻ�����Ļ�ѧ���Ƿ�ȫ���ǹ��ۼ�
D����ѧ�仯���Ƿ�����ЧӦ����ɫ�仯�������������ɵ�����ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na����NH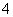 ��Mg2����Al3����SO
��Mg2����Al3����SO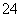 ��NO
��NO ��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ����������ʵ�飺
��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ����������ʵ�飺
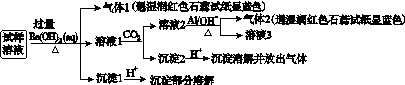
��֪��3NO ��8Al��5OH����2H2O
��8Al��5OH����2H2O 3NH3����8AlO
3NH3����8AlO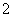
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����(����)
A�������п϶�����NH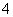 ��Mg2����SO
��Mg2����SO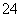 ��NO
��NO
B��������һ������Al3��
C�������п��ܴ���Na����Cl��
D���������п��ܴ���NaNO3��NH4Cl��MgSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
(1)��ԭ������������˳��(ϡ���������)������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
(2)ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
(3)��֪��
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����__________________________________��
����ʱ�����Al2O3�������AlCl3��ԭ����______________________________��
(4)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
Si(��)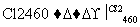 SiCl4
SiCl4 SiCl4(��)
SiCl4(��)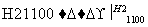 Si(��)
Si(��)
д��SiCl4�ĵ���ʽ��________________����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(5)P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 ��b��HI c��SO2 d��CO2
(6)KClO3������ʵ������O2�������Ӵ�����400 ��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
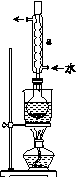
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)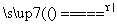 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O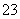 ��I2===S4O
��I2===S4O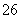 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
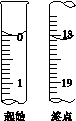
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO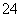 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����仯�����������������ϵ���С�
(1)��ͼ��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ��
�ٸõ绯��ʴ��Ϊ________��
��ͼ��A��B��C��D�ĸ�������������������________(����ĸ)��
(2)�÷���Ƥ��ȡ����(Fe2O3)�IJ�������ʾ��ͼ���£�
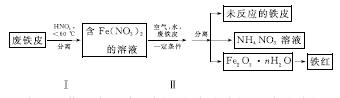
�ٲ�������¶ȹ��ߣ�����������ֽ⡣����ֽ�Ļ�ѧ����ʽΪ______________________________��
�ڲ�����з�����Ӧ��4Fe(NO3)2��O2��(2n��4)H2O===2Fe2O3��nH2O��8HNO3����Ӧ������HNO3�ֽ�����Ƥ�е���ת��ΪFe(NO3)2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
���������������У������֡���ɫ��ѧ��˼�����______(��дһ��)��
(3)��֪t ��ʱ����ӦFeO(s)��CO(g)Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
��t ��ʱ����Ӧ�ﵽƽ��ʱn(CO)��n(CO2)��________��
������1 L�ܱ������м���0.02 mol FeO(s)����ͨ��x mol CO, t ��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO(s)ת����Ϊ50%����x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʷ�����ѧ��Ӧ���仯ѧ��Ӧ�������ڼӳɷ�Ӧ����(����)
A������������ B����ϩ���Ȼ���
C���Ҵ���Ũ���� D�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������£�һ���ܴ����������������(����)
A����ɫ����ˮ��Һ�У�K����Ba2����I����MnO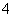
B�����д���NO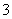 ��ˮ��Һ�У�NH
��ˮ��Һ�У�NH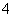 ��Fe2����SO
��Fe2����SO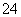 ��H��
��H��
C��c(HCO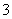 )��0.1 mol/L����Һ�У�Na����K����CO
)��0.1 mol/L����Һ�У�Na����K����CO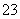 ��Br��
��Br��
D��ǿ������Һ�У�ClO����S2����HSO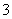 ��Na��
��Na��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com