
��21�֣�ʵ����������80mL��1.5 mol/L��NaHCO3��Һ���Իش�
��1����ʵ�����ʹ�õIJ������� ��
��2�����ø���ҺʱӦ��ȡNaHCO3������Ϊ_____________________����3�����в���������������Һ���ʵ���Ũ�ȵ�Ӱ�죨��д��Ӱ�졢ƫ��ƫ�ͣ�
A�����ƹ�����δϴ���ձ��Ͳ�������
B������ƿʹ��֮ǰδ��ɣ�����������ˮ��
C������ʱ��������ƿ�Ŀ̶��ߣ�
D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦����__________
д�����з�Ӧ�����ӷ���ʽ��
A����NaHCO3��Һ�еμ�����
B����Ba(OH)2��Һ�еμ�����NaHCO3��Һ
C����ˮ�еμ�MgCl2��Һ
��1���ձ�������������ͷ�ιܡ�100ml����ƿ����2��12.6g����3�� A��ƫ��
B����Ӱ�� C��ƫ�� D����Ӱ��
��4�� A��HCO3-+H+=H2O+CO2�� B��HCO3-+Ba2++OH-=BaCO3��+H2O
C��2NH3��H2O+Mg2+=Mg(OH)2��+2NH4+
���������������1����ʵ�����ʹ�õIJ������У��ձ�������������ͷ�ιܡ�100ml����ƿ��
��2��Ҫ����80mL��1.5 mol/L��NaHCO3��Һ��ʵ���ϸ�������ƿ�Ĺ��Ҫ����100��������Һ���ʳ�ȡNaHCO3������Ϊ0.1��1.5��84= 12.6g����3��A�����ƹ�����δϴ���ձ��Ͳ��������ձ��Ͳ�������մ�����ʣ��������Ƶ���Һ��Ũ��ƫ�ͣ�B������ƿʹ��֮ǰδ��ɣ�����������ˮ����Ӱ�������Һ�����������Ӱ�죻C������ʱ��������ƿ�Ŀ̶��ߣ���Һ�����ƫС����Ũ��ƫ�ߣ�D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦������Һ���٣�����Ӱ��Ũ�ȡ���4��A����NaHCO3��Һ�еμ����̼�����Ʋ�������Ӻ�̼��������ӣ������������Ӻ������ӣ���Ӧ�����Ȼ��Ʋ�����ӣ������ӷ���ʽд�ɣ�HCO3-+H+=H2O+CO2����B����Ba(OH)2��Һ�еμ�����NaHCO3��Һ����Ӧ����̼�ᱵ���������������ƣ������ӷ���ʽд�ɣ�HCO3-+Ba2++OH-=BaCO3��+H2O��C����ˮ�еμ�MgCl2��Һ����Ӧ����������þ���������Ȼ�泥������ӷ���ʽд��2NH3��H2O+Mg2+=Mg(OH)2��+2NH4+��
���㣺����һ�����ʵ���Ũ�ȵ���Һ�������������ӷ���ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������ҹ�������н�ֹ����ʹ�ú�Ǧ���ͣ�����Ҫԭ����
| A���������ȼ��Ч�� | B���������ͳɱ� |
| C������Ǧ��Ⱦ���� | D��Ǧ��Դ��ȱ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ೱ���ҹ�ʱ�з��������ೱ����ʱ����ˮ�е�ijЩС�������������ֳ��ʹˮ�ʺ졢�ϵ���ɫ�������������Σ��������˵������ȷ����
| A���ೱ��ˮ�帻Ӫ�����Ľ�� |
| B������ϴ�Ӽ��Ĺ㷺Ӧ�����ŷ��Ƿ����ೱ����Ҫԭ�� |
| C���ڷ�յĺ���������ೱ |
| D���ೱ�ķ������������ص���Ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
6��)��Һ��������ѧ��ѧʵ��Ļ���������
��1�����������ƹ�������100g10��������������Һʱ����Ҫ�õ��IJ��������в������� __________��
��2������һ�����ʵ���Ũ����Һʱ�õ��������϶࣬�����й�������ȷ����_____��
a.����NaOH����ʱ���ֱ�����ƽ���������̵�����ͬ��С��ֽƬ
b���������������ǽ��衢����
c����Ũ��������ϡ��Һʱ������Ͳ��ϡ�ͺ�Ҫ��ȴ��������ת�Ƶ�����ƿ��
d������ƿ��ʹ��ǰҪ����Ƿ�©ˮ
��3��ʵ��������һ�����ʵ���Ũ����Һ�����������ݲ����ľ��巽����������ƿ��ע������ˮ����̶���1~2cm����Ȼ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��20�֣����к�������NaCl��Na2SO4��Na2CO3�����ʵ�NaNO3��Һ��ѡ���ʵ����Լ���ȥ���ʣ��õ�������NaNO3���壬ʵ����������ͼ��ʾ��
��1������A����Ҫ�ɷ��� ���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ�� ��
��3���٢ڢ��о����еķ�������� ��
��4����Һ3�����������Եõ�NaNO3���壬��Һ3�п϶����е������� ��Ϊ�˳�ȥ���ʣ�������Һ3�м��������� ��
��5��ʵ����������ʵ���õ�NaNO3��������500 mL 0.40 mol/L NaNO3��Һ��
��������Һʱ���������²�����a�����ݣ�b�����㣻c���ܽ⣻d��ҡ�ȣ�e��ת�ƣ�f��ϴ�ӣ�j����������ȡNaNO3����������� g��
���ղ���˳��4���� ������ţ���
��ijͬѧת����Һ�IJ�����ͼ��ʾ����ͬѧ�����еĴ����� ��
�����ý�ͷ�ιܶ���ʱ����С�ĵ�ˮ�ι��˿̶��ߣ�����ΪӦ�ò�ȡ�Ĵ��������ǣ� ��
�����в����У����������������Һ��Ũ��ƫ�͵��� ����ѡ���
a��û��ϴ���ձ��Ͳ�����
b������ʱ�����ӿ̶���
c��ϴ�Ӻ������ƿ�в�����������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
15��)ʵ�������������ƹ�������1��0 mol/L��NaOH��Һ500 mL���ش��������⣺
��1�����Ҫ������ʵ�����Ҫʵ�鲽�裺
��__________________����__________________��
��__________________����__________________��
��__________________����__________________��
��2����������Ϊ������ƿ(���Ϊ________)��������ƽ������Ҫ��Щʵ������������ɸ�ʵ�飬��д����_________________________________________
��3�����в�����������Һ��Ũ���к�Ӱ�죿(��д��ĸ)
ƫ�����_______________��ƫС���� ����Ӱ����� ��
| A������ʱʹ������������� |
| B����NaOH����ֽ���ϳ��� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�� |
| D��������ƿ����Һʱ��������Һ�彦�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�ʵ�������ܶ�Ϊ1��18g/mL����������Ϊ36��5%Ũ��������250mLO��lmol/L��ϡ������Һ��ղ���ش��������⣺
��1��ʹ������ƿǰ������е�һ��������______________________________��
��2������250mL0��lmol/L����ϡ����Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________________________________��
| A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ���� |
| B������Ͳȷ��ȡ����Ũ�������__________mL���ز����������ձ��У��ټ�������ˮ(Լ30mL)���ò���������������ʹ���Ͼ��� |
| C��������ȴ�������ز�����ע��mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��13�֣���ʾ��ҺŨ�ȵķ���ͨ��������:��Һ�����ʵ���������(��)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������������⡣
��1����10��(�ܶ�Ϊ1.00g��cm-3)��NaOH��Һ���Ƴ�27.5g2����NaOH��Һ��
�ټ��㣺��______g10��(�ܶ�Ϊ1.00g��cm-3)��NaOH��Һ�����______mLˮ(�ܶ�Ϊ1.00g��cm-3)����ϡ�͡�
����ȡ����_______mL��Ͳ���ɹ�ѡ�����Ͳ����У�5mL��10mL��25mL��50mL����ͬ����ȡ10����NaOH��Һ����ȡʱ����Ҫ����Ͳ��Һ��_______���У�Ȼ�����ձ����______mL��Ͳ��ȡ����ˮע���ձ��
��2����98��(�ܶ�Ϊ1.84g��cm-3)��Ũ����ϡ�ͳ�3mol/L��ϡ����100mL���ش��������⣺
����Ҫ��ȡŨ����___________mL(����һλС��)��
�����Ʋ����ɷֽ�����¼�������ȷ�IJ���˳���ǣ�___________��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ�������������ϴ��Һע������ƿ�У����ظ���������
C��������ȴ��ϡ����ע�뾭��鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ�����ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹҺ��ﵽ�̶���
H������������ƿ�м�����ˮ��ʹҺ��ӽ��̶���
���������C�����а�δ��ȴ��ϡ����ע������ƿ�У�������Һ��Ũ�Ƚ�_________ (�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)���������D��������ȡŨ�������Ͳ��������Ũ��������������ˮϴ�Ӳ���ϴ��Һת��E�����е�С�ձ��У�������Һ��Ũ�Ƚ�_________���������G������Ŀ�����ӣ�������Һ��Ũ�Ƚ�_________���������D������Ŀ�⸩�ӣ�������Һ��Ũ�Ƚ�_________��
��3��ʵ������NaOH��������1mol/L��NaOH��Һ����98��(�ܶ�Ϊ1.84g��cm-3)��Ũ��������1mol/L��H2SO4��Һ��100mL��
������1mol/L��NaOH��Һ������������ƽ��ȡNaOH����ʱ����ƽ����Ϊ_______(����ţ���ͬ)��
A��4.0g B����4.0g C������4.0g
������1mol/L��H2SO4��Һ�����ձ���ϡ��Ũ�������ȷ�����ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��8�֣�Ϊ��֤������Cl2 > Fe3�� > SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г�������A�м���װ�����ԣ��������Ѽ��飩��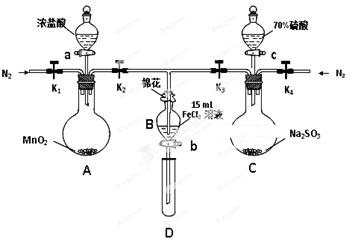
ʵ����̣�
I. ���ɼ�K1~K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��. ����a���μ�һ������Ũ���ᣬ��A���ȡ�
��. ��B����Һ���ʱ��ֹͣ���ȣ��ر�K2��
��. ����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��. ��K3�ͻ���c������70%�����ᣬһ��ʱ���ر�K3��
��. �����Թ�D���ظ����̢�������B��Һ�е����ӡ�
��1�����̢��Ŀ���� ��
��2�����н������ҺΪ ��
��3��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��4��������ȡ��SO2ͨ�����Ը��������Һ��ʹ��Һ��ɫ�������ӷ���ʽΪ ��
��5���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬�������±���ʾ�����ǵļ����һ���ܹ�֤��������Cl2 > Fe3�� > SO2���� ����ס����ҡ�����������
| | ���̢�B��Һ�к��е����� | ���̢�B��Һ�к��е����� |
| �� | ��Fe3����Fe2�� | ��SO42�� |
| �� | ����Fe3������Fe2�� | ��SO42�� |
| �� | ��Fe3����Fe2�� | ��Fe2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com