
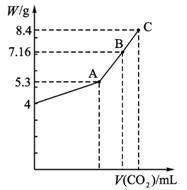
Ķ¼1-5-26
(1)AµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ__________£¬ĶØČėCO2µÄĢå»żĪŖ__________mL”£
(2)CµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ__________£¬ĶØČėCO2µÄĢå»żĪŖ__________mL”£
(3)ĶĘĖćBµćŹ±MµÄ×é³É(ÓĆ»ÆѧŹ½±ķŹ¾)¼°ĶØČėCO2ĘųĢåµÄĢå»ż”£
½āĪö:(1)ÓÉĶ¼æÉÖŖNaOHÖŹĮæĪŖ4 g£¬ĪļÖŹµÄĮæĪŖ0.1 mol£¬ĶźČ«×Ŗ»ÆĪŖNa2CO3Ź±£¬Ģ¼ĖįÄʵÄÖŹĮæĪŖ5.3 g£¬ŠčCO2Ģå»żĪŖ![]() ”Į22.4 L”¤mol-1=1.12 L”£
”Į22.4 L”¤mol-1=1.12 L”£
(2)ĶźČ«×Ŗ»ÆĪŖNaHCO3Ź±£¬NaHCO3ÖŹĮæĪŖ8.4 g£¬ŠčCO2µÄĢå»żĪŖ0.1 mol”Į22.4 L”¤mol-1=2.24 L”£?
(3)Ķ¼ÖŠBµćŹ±MµÄÖŹĮæĪŖ7.16 g,5.3£¼7.16£¼8.4,ÖŖMÓÉNa2CO3ŗĶNaHCO3×é³É”£ÉčŌŚBµćŹ±Na2CO3µÄĪļÖŹµÄĮæĪŖx£¬NaHCO3µÄĪļÖŹµÄĮæĪŖy£¬ŌņÓŠ£ŗ
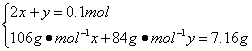 ½āµĆ
½āµĆ![]()
V(CO2)=(0.02+0.06) mol”Į22 400 mL”¤mol-1=1 792 mL
“š°ø:(1)Na2CO3 1 120 mL (2)NaHCO3 2 240 mL (3)Na2CO3”¢NaHCO3 1 792 mL


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A.150 mL B.200 mL C.300 mL D.350 mL
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
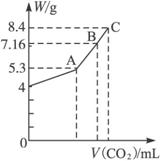
Ķ¼7-3
£Ø1£©AµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ______________£¬ĶØČėCO2µÄĢå»żĪŖmL______________”£
£Ø2£©CµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ______________£¬ĶØČėCO2µÄĢå»żĪŖmL______________”£
£Ø3£©ĶĘĖćBµćŹ±MµÄ×é³É£ØÓĆ»ÆѧŹ½±ķŹ¾£©¼°ĶØČėCO2ĘųĢåµÄĢå»ż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ¼ŖĮÖŹ”³¤“ŗŹŠŹµŃé֊ѧøßČżÉĻѧʌµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø8·Ö£©Ķł100 mLµÄNaOHČÜŅŗÖŠĶØČėCO2³ä·Ö·“Ó¦ŗó£¬ŌŚ¼õŃ¹ŗĶ½ĻµĶĪĀ¶ČĻĀ£¬Š”ŠÄµŲ½«ČÜŅŗÕōøÉ£¬µĆµ½°×É«¹ĢĢåM”£ĶØČėµÄCO2µÄĢå»żV(±ź×¼×“æö)ÓėMµÄÖŹĮæWµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£ŹŌ½ā“šĻĀĮŠĪŹĢā£ŗ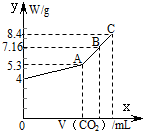
(1)AµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ____________________£¬ĶØČėµÄCO2µÄĢå»żĪŖ________ mL(±ź×¼×“æöĻĀ£¬ĻĀĶ¬)”£
(2)CµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖ____________________£¬ĶØČėµÄCO2µÄĢå»żĪŖ________ mL”£
(3)BµćŹ±MµÄ×é³É³É·ÖĪŖ________(ÓĆ»ÆѧŹ½±ķŹ¾)£¬ĶØČėµÄCO2µÄĢå»żĪŖ________ mL”£
(4)øĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½ĖÕŹ”ÄĻ¾©Ń§“ó½ĢÓż×ØŠŽŃ§Š£øßŅ»4ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗµ„Ń”Ģā
Ķł100 mL 1mol/LµÄAlCl3ČÜŅŗÖŠµĪ¼Ó1mol/LµÄNaOHČÜŅŗ£¬µĆµ½5.85g³Įµķ£¬Ōņ¼ÓČėNaOHČÜŅŗµÄĢå»żæÉÄÜŹĒ
| A£®200 mL | B£®225mL | C£®300 mL | D£®325 mL |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com