
ijĶ¬Ń§ŌŚŃ§Ļ°¹ż³ĢÖŠĮĖ½āµ½µ°æĒµÄÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįøĘ”£ÓŚŹĒ£¬øĆĶ¬Ń§ÉčĻėŹĒ²»ŹĒĖłÓŠµ°æĒÖŠµÄŗ¬øĘĮ涼Ņ»Ńł£¬Čē¹ū¶¼Ņ»ŃłµÄ»°£¬“óŌ¼ŹĒ¶ąÉŁ£æČē¹ū²»Ņ»ŃłµÄ»°£¬µ°æĒÖŠµÄŗ¬øĘĮæÓėŹ²Ć“ŅņĖŲÓŠ¹ŲÄŲ£æÓŚŹĒøĆĶ¬Ń§¾ĶŅŌ“ĖĪŖæĪĢāÓėĘäĖūĶ¬Ń§Ņ»ĘšæŖÕ¹ĮĖŅ»“ĪŃŠ¾æŠŌѧĻ°”£ŌŚŃŠ¾æŠŌѧĻ°ÖŠ£¬øĆĶ¬Ń§µÄ·Ö¹¤ŹĒÉč¼Ę²āĮæµ°æĒÖŠŗ¬øĘĮæµÄ·½°ø”£
ĻĀĆęŹĒĖūÉč¼ĘµÄŅ»øö·½°ø£¬ĒėÄćĪŖĖū²¹Č«·½°ø£¬²¢»Ų“šĻą¹ŲĪŹĢā”£
µŚŅ»²½£ŗŹÕ¼Æµ°æĒ£¬²¢Ļ“µÓøɾ»±øÓĆ”£
µŚ¶ž²½£ŗ³ĘĮæµ°æĒÖŹĮæ(m1)”£
µŚČż²½£ŗČܽāµ°æĒ”£½«µ°æĒ·ÅČėŹ¢ÓŠ________µÄÉÕ±ÖŠ£¬½Į°č£¬Ź¹Ęä³ä·Ö·“Ó¦£¬¹żĀĖ³żČ„²»ČÜŠŌŌÓÖŹ£¬±£ĮōĀĖŅŗ±øÓĆ”£µ°æĒČܽā¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________”£
µŚĖIJ½£ŗ³ĮµķCa2£«”£ĻņĀĖŅŗÖŠ¼ÓČė¹żĮæµÄ________ČÜŅŗ”£
µŚĪå²½£ŗ¹żĀĖ£¬Ļ“µÓ³Įµķ”£
µŚĮł²½£ŗ³ĘĮæ³ĮµķÖŹĮæ(m2)”£
µ°æĒÖŠŗ¬øĘĮæ(¼“øĆŌŖĖŲµÄÖŹĮæ·ÖŹż)µÄ±ķ“ļŹ½ĪŖw(Ca)£½
__________ӣ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½šŹōŅ±Į¶Óė“¦Ąķ³£Éę¼°Ńõ»Æ»¹Ō·“Ó¦”£
(1)ÓÉĻĀĮŠĪļÖŹŅ±Į¶ĻąÓ¦½šŹōŹ±²ÉÓƵē½ā·ØµÄŹĒ________”£[Ą“Ō“:Z#xx#k.Com]
a£®Fe2O3 b£®NaCl
c£®Cu2S d£®Al2O3[Ą“Ō“:ѧ,æĘ,ĶųZ,X,X,K]
(2)»ŌĶæó(Cu2S)æÉ·¢Éś·“Ó¦£ŗ2Cu2S£«2H2SO4£«5O2===4CuSO4£«2H2O£¬øĆ·“Ó¦µÄ»¹Ō¼ĮŹĒ________”£µ±1 mol O2·¢Éś·“Ó¦Ź±£¬»¹Ō¼ĮĖłŹ§µē×ÓµÄĪļÖŹµÄĮæĪŖ________mol”£ĻņCuSO4ČÜŅŗÖŠ¼ÓČėĆ¾ĢõŹ±ÓŠĘųĢåÉś³É£¬øĆĘųĢåŹĒ________”£
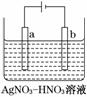
(3)ÓŅĶ¼ĪŖµē½ā¾«Į¶ŅųµÄŹ¾ŅāĶ¼£¬________(Ģī”°a”±»ņ”°b”±)¼«ĪŖŗ¬ÓŠŌÓÖŹµÄ“ÖŅų£¬Čōb¼«ÓŠÉŁĮæŗģ×ŲÉ«ĘųĢå²śÉś£¬ŌņÉś³ÉøĆĘųĢåµÄµē¼«·“Ó¦Ź½ĪŖ__________________________”£
(4)ĪŖ“¦ĄķŅųĘ÷±ķĆęµÄŗŚ°ß(Ag2S)£¬½«ŅųĘ÷½žÓŚĀĮÖŹČŻĘ÷ĄļµÄŹ³ŃĪĖ®ÖŠ²¢ÓėĀĮ½Ó“„£¬Ag2S×Ŗ»ÆĪŖAg£¬Ź³ŃĪĖ®µÄ×÷ÓĆŹĒ______________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚ±½µÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ (””””)
A£®±½µÄ·Ö×ÓŹ½ĪŖC6H6£¬Ėü²»ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬ŹōÓŚ±„ŗĶĢž
B£®“Ó±½µÄææāĄÕŹ½(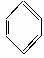 )æ“£¬±½·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬Ó¦ŹōÓŚĻ©Ģž
)æ“£¬±½·Ö×ÓÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬Ó¦ŹōÓŚĻ©Ģž
C£®ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬±½ÓėŅŗäå·“Ó¦Éś³Éäå±½£¬·¢ÉśĮĖ¼Ó³É·“Ó¦
D£®±½·Ö×ÓĪŖĘ½ĆęÕżĮł±ßŠĪ½į¹¹£¬6øöĢ¼Ō×ÓÖ®¼äµÄ¼üĶźČ«ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ÖŠAŹĒÖĘČ”äå±½µÄŹµŃé×°ÖĆ£¬B”¢CŹĒøĽųŗóµÄ×°ÖĆ£¬Ēė׊Ļø·ÖĪö£¬¶Ō±ČČżøö×°ÖĆ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
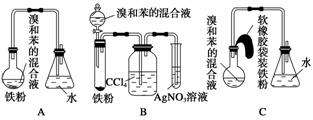
(1)Š“³öČżøö×°ÖĆÖŠĖł¹²Ķ¬·¢ÉśµÄĮ½øö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_____________________________________________________________£»
_____________________________________________________________ӣ
Š“³öBÖŠŹ¢ÓŠAgNO3ČÜŅŗµÄŹŌ¹ÜÖŠĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______________________________________________________________”£
(2)×°ÖĆAŗĶC¾ł²ÉÓĆĮĖ³¤²£Į§µ¼¹Ü£¬Ęä×÷ÓĆŹĒ__________________________
________________________________________________________________ӣ
(3)ŌŚ°“×°ÖĆB”¢C×°ŗĆŅĒĘ÷¼°Ņ©Ę·ŗóŅŖŹ¹·“Ó¦æŖŹ¼£¬Ó¦¶Ō×°ÖĆB½ųŠŠµÄ²Ł×÷ŹĒ_____________________________________________________________£»
Ó¦¶Ō×°ÖĆC½ųŠŠµÄ²Ł×÷ŹĒ_______________________________________”£
(4)×°ÖĆB”¢C½ĻŗƵŲ½ā¾öĮĖAÖŠ¼Ó×°Ņ©Ę·ŗĶŹ¹×°ÖĆ¼°Ź±ĆÜ·āµÄƬ¶Ü£¬·½±ćĮĖ²Ł×÷”£A×°ÖĆÖŠÕāŅ»ĪŹĢāŌŚŹµŃéÖŠŌģ³ÉµÄŗó¹ūŹĒ________________________”£
(5)BÖŠ²ÉÓĆĮĖĻ“ĘųĘæĪüŹÕ×°ÖĆ£¬Ęä×÷ÓĆŹĒ_____________________________£¬
·“Ó¦ŗóĻ“ĘųĘæÖŠæÉÄܳöĻÖµÄĻÖĻóŹĒ__________________________________”£
(6)B×°ÖĆŅ²“ęŌŚĮ½øöĆ÷ĻŌµÄȱµć£¬Ź¹ŹµŃéµÄŠ§¹ū²»ŗĆ»ņ²»ÄÜÕż³£½ųŠŠ”£ÕāĮ½øöȱµćŹĒ________________________________________________________
_______________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ(””””)
A£®Šæʬ²åČėĻõĖįŅųČÜŅŗÖŠZn£«Ag£«===Zn2£«£«Ag
B£®Ģ¼ĖįĒāøĘČÜŅŗ¼Óµ½“×ĖįÖŠ
Ca(HCO3)2£«2CH3COOH===Ca2£«£«2CH3COO££«2CO2”ü£«2H2O
C£®ÉŁĮ潚ŹōÄĘ¼Óµ½ĄäĖ®ÖŠ
Na£«2H2O===Na£«£«OH££«H2”ü
D£®ĒāŃõ»ÆĶ¼Óµ½ŃĪĖįÖŠ
Cu(OH)2£«2H£«===Cu2£«£«2H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ”°“ÖŃĪĢį“æ”±ŹµŃéÖŠ,Õō·¢Ź±ÕżČ·µÄ²Ł×÷ŹĒ(””””)
A.°Ń»ė×ĒµÄŅŗĢåµ¹ČėÕō·¢ĆóÄŚ¼ÓČČ
B.æŖŹ¼Īö³ö¾§ĢåŗóÓĆ²£Į§°ō½Į°č
C.“żĖ®·ÖĶźČ«ÕōøÉŗóĶ£Ö¹¼ÓČČ
D.Õō·¢ĆóÖŠ³öĻÖ½Ļ¶ąĮæ¹ĢĢåŹ±¼“Ķ£Ö¹¼ÓČČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
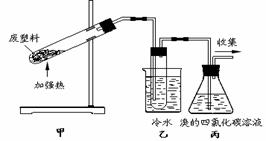
¼ÓČČ¾Ū±ūĻ©·ĻĖÜĮĻæÉŅŌµĆµ½Ģ¼”¢ĒāĘų”¢¼×Ķ锢ŅŅĻ©”¢±ūĻ©”¢±½ŗĶ¼×±½”£ÓĆĶ¼ĖłŹ¾×°ÖĆĢ½¾æ·Ļ¾ÉĖÜĮĻµÄŌŁĄūÓĆ”£ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ
A£®¾Ū±ūĻ©µÄĮ“½ŚŹĒ”ŖCH2”ŖCH2”ŖCH2”Ŗ
B£®×°ÖĆŅŅµÄŹŌ¹ÜÖŠæÉŹÕ¼Æµ½·¼ĻćĢž
C£®×°ÖƱūÖŠæɵƵ½Ā±“śĢž
D£®×īŗóŹÕ¼ÆµÄĘųĢåæÉ×öČ¼ĮĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æģĄÖŹĒŹ²Ć“£æ¾«Éń²”ѧ×ؼŅĶعżŹµŃé·¢ĻÖ£ŗŌŚ“óÄŌµÄĻąÓ¦²æĪ»”Ŗ£”°½±ÉĶÖŠŠÄ”±£¬øųÓčČįŗĶµÄµē»÷£¬±ć»įŹ¹“óÄŌ“¦ÓŚ¼«¶ČæģĄÖµÄדĢ¬”£ČĖĆĒŅŃ¾½«”°½±ÉĶÖŠŠÄ”±ø÷²æ·ÖµÄÄŌµēĶ¼»ęÖĘ³öĄ“£¬²¢ČĻĪŖ£¬ŌŚø÷ĒųÓņÖ®¼ä“«µŻŠÅĻ¢µÄ»ÆѧĪļÖŹŹĒ¶ą°Ķ°·£¬ĖłŅŌ”°½±ÉĶÖŠŠÄ”±ÓÖ³ĘĪŖ¶ą°Ķ°·ĻµĶ³”£¶ą°Ķ°·½į¹¹ČēĻĀĶ¼£ŗ
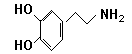 ¢ÅŹŌÅŠ¶Ļ¶ą°Ķ°·ÄÜ·¢ÉśµÄ»Æѧ·“Ó¦ ”£
¢ÅŹŌÅŠ¶Ļ¶ą°Ķ°·ÄÜ·¢ÉśµÄ»Æѧ·“Ó¦ ”£
A£®¼Ó³É B£®ĻūČ„ C£®Ńõ»Æ D£®Ė®½ā
¢ĘŠ“³öÓė¶ą°Ķ°·»„ĪŖĶ¬·ÖŅģ¹¹ĢåĒŅŹōÓŚ1£¬3£¬5£ČżČ”“ś±½²¢ĒŅ±½»·ÉĻÖ±½ÓĮ¬ÓŠŅ»øöōĒ»łŗĶŅ»øö°±»łĒŅ·Ö±šÄÜÓėÄĘŗĶĒāŃõ»ÆÄĘ·“Ó¦£¬ĻūŗÄÄĘÓėĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæÖ®±ČĪŖ£²£ŗ£±µÄĖłÓŠĪļÖŹµÄ½į¹¹¼ņŹ½£ŗ
ӣ
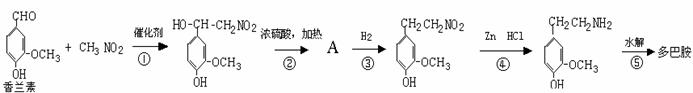 ¢Ē¶ą°Ķ°·æÉÓÉĻćĄ¼ĖŲÓėĻõ»ł¼×ĶéĖõŗĻ£¬ŌŁ¾Šæ£ŃĪĖį»¹ŌĖ®½ā¶ųµĆ”£ŗĻ³É¹ż³Ģ±ķŹ¾ČēĻĀ£ŗ
¢Ē¶ą°Ķ°·æÉÓÉĻćĄ¼ĖŲÓėĻõ»ł¼×ĶéĖõŗĻ£¬ŌŁ¾Šæ£ŃĪĖį»¹ŌĖ®½ā¶ųµĆ”£ŗĻ³É¹ż³Ģ±ķŹ¾ČēĻĀ£ŗ

ĒėŠ“³ö¢Ś”¢¢ŻĮ½²½µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ś£ŗ
¢Ż£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪļÖŹ×Ŗ»ÆŌŚøų¶ØĢõ¼žĻĀÄÜŹµĻֵďĒ(””””)
A£®Al2O3 NaAlO2(aq)
NaAlO2(aq)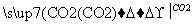 Al(OH)3
Al(OH)3
B£®S SO3
SO3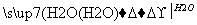 H2SO4
H2SO4
C£®±„ŗĶNaCl(aq)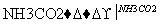 NaHCO3
NaHCO3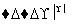 Na2CO3
Na2CO3
D£®Fe2O3 FeCl3(aq)
FeCl3(aq)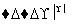 ĪŽĖ®FeCl3
ĪŽĖ®FeCl3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com