
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ǽ����������в������ݣ�CO32-+2H+�TCO2��+H2O |
| B�����������������ܽ���ϡ������Һ�У�Fe3O4+8H+�T2Fe3++Fe2++4H2O |
| C��������ͨ��������Һ�У�NH3+H+�TNH4+ |
| D����̼�������Һ�м���������NaOH��Һ��NH4++OH-�TNH3?H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ϊ����ԭ�ϣ�������Ӧ�õ�һϵ�еķ�Ȳ��������ṹΪ��
��Ϊ����ԭ�ϣ�������Ӧ�õ�һϵ�еķ�Ȳ��������ṹΪ��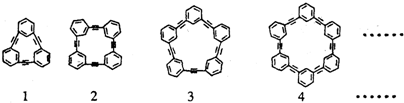
 ��Ϊ��ʼ���ʣ�ͨ���ӳɡ���ȥ��Ӧ�Ƶã�д���ɱ���ϩ��ȡ����Ȳ�Ļ�ѧ����ʽ����������Լ���ѡ�����ԣ��������ɣ�
��Ϊ��ʼ���ʣ�ͨ���ӳɡ���ȥ��Ӧ�Ƶã�д���ɱ���ϩ��ȡ����Ȳ�Ļ�ѧ����ʽ����������Լ���ѡ�����ԣ��������ɣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ | B����С |
| C������ | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H+��Cl-��Na+��I- |
| B��Mg2+��NO3-��K+��Br- |
| C��H+��Na+��CO32-��Cl- |
| D��Ag+��NO3-��Na+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����״���£��ܶ�Ϊdg/L��ij���崿����һ�����ӵ�����Ϊ
| ||
| B��1 mol FeCl3��ˮ��Ӧ��ȫת��������������������ɽ������ӵ���ĿΪNA | ||
| C��31g�������У����еĹ��۵�����Ŀ��NA�� | ||
| D�����CuCl2��Һʱ������NA������ͨ��������������64g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com