
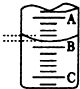
 ²ŻĖį£ØH2C2O4£©“ęŌŚÓŚ×ŌČ»½ēµÄÖ²ĪļÖŠ£¬ĘäK1=5.4”Į10-2£¬K2=5.4”Į10-5£¬¾ßÓŠ»¹ŌŠŌ£¬ČÜÓŚĖ®£¬ČÜŅŗÓŠĖįŠŌ£¬ĪŖ²ā¶ØijH2C2O4ČÜŅŗµÄÅØ¶Č£¬Č”øĆČÜŅŗӌ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæĻ”H2SO4ŗó£¬ÓĆÅضČĪŖc mol/L KMnO4±ź×¼ČÜŅŗµĪ¶Ø£®µĪ¶ØŌĄķĪŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2”ü+2MnSO4+8H2O
²ŻĖį£ØH2C2O4£©“ęŌŚÓŚ×ŌČ»½ēµÄÖ²ĪļÖŠ£¬ĘäK1=5.4”Į10-2£¬K2=5.4”Į10-5£¬¾ßÓŠ»¹ŌŠŌ£¬ČÜÓŚĖ®£¬ČÜŅŗÓŠĖįŠŌ£¬ĪŖ²ā¶ØijH2C2O4ČÜŅŗµÄÅØ¶Č£¬Č”øĆČÜŅŗӌ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæĻ”H2SO4ŗó£¬ÓĆÅضČĪŖc mol/L KMnO4±ź×¼ČÜŅŗµĪ¶Ø£®µĪ¶ØŌĄķĪŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2”ü+2MnSO4+8H2O| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄKMnO4ČÜŅŗĢå»ż/mL | 22.32 | 24.39 | 24.41 |
·ÖĪö £Ø1£©ŹµŃéĒ°µĪ¶Ø¹Ü±ŲŠė½ųŠŠ¼ģĀ©£»KMnO4ČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬æÉŅŌøÆŹ“ĻšĘ¤¹Ü£»KMnO4ČÜŅŗ³Ź×ĻÉ«£¬²ŻĖį·“Ó¦Ķź±Ļ£¬µĪČė×īŗóŅ»µĪKMnO4ČÜŅŗ£¬ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓ²»ĶŹÉ«ĪŖµĪ¶Øµ½ÖÕµć£»
£Ø2£©ČōAÓėCæĢ¶Č¼äĻą²ī1mL£¬ĖµĆ÷ĆæĮ½øöŠ”øńÖ®¼äŹĒ0.1mL£¬C“¦µÄæĢ¶ČĪŖ20£¬¾Ż“ĖČ·¶ØAµÄæĢ¶Č£¬×¢ŅāµĪ¶Ø¹ÜµÄÉĻĆꏿֵŠ”£¬ĻĀĆꏿֵ“󣬾ą»īČūÓŠŅ»¶Ī¾ąĄėƻӊæĢ¶Č£»
£Ø3£©øł¾ŻĖłÓĆ¹ż³ĢÅŠ¶Ļ²»µ±²Ł×÷¶ŌĻą¹ŲĪļĄķĮæµÄÓ°Ļģ£»
£Ø4£©øł¾Ż»Æѧ·½³ĢŹ½2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2”ü+2MnSO4+8H2O¼ĘĖć²ŻĖįµÄÅØ¶Č£»
£Ø5£©øł¾ŻĒæĖįÖĘČõĖįµÄŌĄķÓĆĒæĖįŗĶČõĖįµÄŃĪ·“Ó¦Ą“½ā“š£»
½ā“š ½ā£ŗ£Ø1£©µĪ¶Ø¹ÜŌŚŹ¹ÓĆÖ®Ē°£¬±ŲŠė½ųŠŠµÄ²Ł×÷ŹĒ¼ģ²éŹĒ·ńĀ©Ė®£»KMnO4ČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬æÉŅŌøÆŹ“ĻšĘ¤¹Ü£¬¹ŹKMnO4ČÜŅŗӦװŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£»
KMnO4ČÜŅŗ³Ź×ĻÉ«£¬²ŻĖį·“Ó¦Ķź±Ļ£¬µĪČė×īŗóŅ»µĪKMnO4ČÜŅŗ£¬ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓ²»ĶŹÉ«ĪŖµĪ¶Øµ½ÖÕµć£¬
¹Ź“š°øĪŖ£ŗĖį£»ĖįŹ½µĪ¶Ø¹Ü£»µĪČė×īŗóŅ»µĪKMnO4ČÜŅŗ£¬ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓ²»ĶŹÉ«£»
£Ø2£©ČōAÓėCæĢ¶Č¼äĻą²ī1mL£¬ĖµĆ÷ĆæĮ½øöŠ”øńÖ®¼äŹĒ0.1mL£¬C“¦µÄæĢ¶ČĪŖ20£¬AŗĶBÖ®¼äŹĒĖÄøöŠ”øń£¬ĖłŅŌĻą²ī0.40mL£¬ŌņBŹĒ19.40mL£¬ÓÉÓŚµĪ¶Ø¹Ü50.00mLæĢ¶ČĻĀ·½»¹ÓŠŅŗĢ壬ĖłŅŌŹµ¼ŹČÜŅŗµÄŅŗĢå“óÓŚ50mL-19.40mL=30.60mL£»
¹Ź“š°øĪŖ£ŗ19.40£»“óÓŚ£»
£Ø3£©A£®ŹµŃé½įŹųŹ±ø©ŹÓæĢ¶ČĻ߶ĮČ”µĪ¶ØÖÕµćŹ±KMnO4ČÜŅŗµÄĢå»ż£¬ŅŗĆęĘ«øߣ¬¶ĮŹżĘ«Š”£¬µ¼ÖĀKMnO4Ģå»żĘ«Š”£¬¹ŹAÕżČ·£»
B£®µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģÓŠĘųÅŻ£¬µĪ¶Ø½įŹųĪŽĘųÅŻ£¬µ¼ÖĀKMnO4Ģå»żĘ«“󣬹ŹB“ķĪó£»
C£®µŚŅ»“ĪµĪ¶ØŹ¢×°±ź×¼ŅŗµÄµĪ¶Ø¹Ü×°ŅŗĒ°ÓĆÕōĮóĖ®ĒåĻ“¹żŗó£¬Ī“ÓƱź×¼ŅŗČóĻ“£¬ČÜŅŗ±»Ļ”ŹĶ£¬KMnO4ÅضČĘ«Š”£¬µ¼ÖĀKMnO4Ģå»żĘ«“󣬹ŹC“ķĪó£»
D£®µŚŅ»“ĪµĪ¶ØÓƵÄ׶ŠĪĘæÓĆ“ż×°ŅŗČóĻ“¹ż£¬ŗóĮ½“ĪĪ“ČóĻ“£¬ČóĻ“׶ŠĪĘæµ¼ÖĀ²ŻĖįµÄĪļÖŹµÄĮæĘ«“󣬵¼ÖĀKMnO4Ģå»żĘ«“󣬹ŹD“ķĪó£»
E”¢µĪ¼ÓKMnO4ČÜŅŗ¹żæģ£¬Ī“³ä·ÖÕńµ“£¬øÕ擵½ČÜŅŗ±äÉ«£¬µ¼ÖĀKMnO4Ģå»żĘ«Š”£¬¹ŹEÕżČ·£»
¹ŹŃ”£ŗAE£®
£Ø4£©Čż“ĪµĪ¶ØĻūŗÄKMnO4ČÜŅŗĢå»ż·Ö±šĪŖ£ŗ22.32mLmL”¢24.39mL”¢24.41mL£¬µŚŅ»“ĪĪó²ī½Ļ“ó£¬ÉįČ„£¬mLmLmLĻūŗÄKMnO4ČÜŅŗĘ½¾łĢå»żĪŖ$\frac{24.39mL+24.41mL}{2}$=24.40mL£¬ĻūŗÄKMnO4ĪļÖŹµÄĮæĪŖn£ØKMnO4£©=cmol/L”Į0.0244L£¬ÓÉ2KMnO4+5H2C2O4+3H2SO4ØTK2SO4+2MnSO4+10CO2”ü+8H2OæÉÖŖ£¬n£ØH2C2O4£©=$\frac{5}{2}$n£ØKMnO4£©=$\frac{5}{2}$”Įcmol/L”Į0.0244L=0.061cmol£¬Ōņ²ŻĖįµÄÅضČĪŖ$\frac{0.061cmol}{V”Į1{0}^{-3}L}$=$\frac{61c}{V}$mol/L£»
¹Ź“š°øĪŖ£ŗ$\frac{61c}{V}$mol/L£»
£Ø5£©Č”ÉŁĮæµÄNaHCO3ÓŚŹŌ¹ÜÖŠ£¬¼ÓČė²ŻĖįČÜŅŗ£¬ÓŠĘųÅŻ²śÉśæÉÖ¤Ć÷²ŻĖįµÄĖįŠŌĒæÓŚĢ¼Ėį£»
¹Ź“š°øĪŖ£ŗȔɣĮæµÄNaHCO3ÓŚŹŌ¹ÜÖŠ£¬¼ÓČė²ŻĖįČÜŅŗ£¬ÓŠĘųÅŻ²śÉś£»
µćĘĄ ±¾ĢāŅŌĪļÖŹŗ¬ĮæĪŖ±³¾°æ¼²éŃõ»Æ»¹ŌµĪ¶ØÓė¼ĘĖć£¬ÄѶČÖŠµČ£¬×¢ŅāŹµŃéŌĄķµÄÕĘĪÕ£¬µĪ¶Ø¹ÜÉĻµÄæĢ¶ČŗĶĮæĶ²ÉĻæĢ¶ČµÄĒų±š£¬ĪŖŅדķµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ļ”ĻõĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£ŗH++OH-=H2O | |
| B£® | ĀĮÓėĻ”ŃĪĖį·“Ó¦£ŗAl+2H+=Al3++H2”ü | |
| C£® | ČżĀČ»ÆĢśČÜŅŗÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£ŗFeCl3+3OH-=Fe£ØOH£©3”ż+3C1- | |
| D£® | ¶žŃõ»ÆĢ¼Óė³ĪĒåŹÆ»ŅĖ®·“Ó¦£ŗCO2+2OH-=CO32-+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
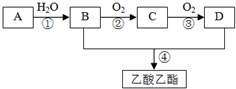 AŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ö£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅĖįŅŅõ„£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£®
AŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ö£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅĖįŅŅõ„£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£® CH3COOCH2CH3+H2O£»
CH3COOCH2CH3+H2O£»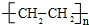 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SiO2$\stackrel{HCl}{”ś}$SiCl4$\stackrel{H_{2}}{”ś}$Si | |
| B£® | MgCO3$\stackrel{HCl}{”ś}$MgCl2ČÜŅŗ$\stackrel{µē½ā}{”ś}$Mg | |
| C£® | Fe$”ś_{µćČ¼}^{O_{2}}$Fe2O3$\stackrel{H_{2}SO_{4}}{”ś}$Fe2£ØSO4£©3 | |
| D£® | Na$”ś_{µćČ¼}^{O_{2}}$Na2O2$\stackrel{CO_{2}}{”ś}$Na2CO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Źµ Ńé ²½ Öč | ĻÖ Ļó | ½į ĀŪ |
| ¢Ł·Ö±šČ”µČĢå»żµÄ2mol/LĮņĖįÓŚŹŌ¹ÜÖŠ£» ¢Ś·Ö±šĶ¶Čė“óŠ””¢ŠĪדĻąĶ¬µÄCu”¢Fe”¢Mg£® | ·“Ó¦æģĀż£ŗ Mg£¾Fe£¾Cu | ·“Ó¦ĪļµÄŠŌÖŹŌ½»īĘĆ£¬·“Ó¦ĖŁĀŹŌ½æģ£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| µĪ¶Ø“ĪŹż | “ż²āŅŗĢå»ż£ØmL£© | ±ź×¼KMnO4ČÜŅŗĢå»ż£ØmL£© | |
| µĪ¶ØĒ°¶ĮŹż | µĪ¶Øŗó¶ĮŹż | ||
| µŚŅ»“Ī | 25.00 | 0.50 | 20.40 |
| µŚ¶ž“Ī | 25.00 | 3.00 | 23.00 |
| µŚČż“Ī | 25.00 | 4.00 | 24.10 |
| ÄŃČÜĪļ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ŃÕÉ« | °× | Ē³»Ę | °× | שŗģ | °× |
| Ksp | 1.77”Į10-10 | 5.35”Į10-13 | 1.21”Į10-16 | 1.12”Į10-12 | 1.0”Į10-12 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃ鱹ŗÅ | ·“Ó¦Īļ | “߻ƼĮ |
| ¢Ł | 10mL2% H2O2ČÜŅŗ | ĪŽ |
| ¢Ś | 10mL5% H2O2ČÜŅŗ | ĪŽ |
| ¢Ū | 10mL5% H2O2ČÜŅŗ | 1mL0.1mol•L-1FeCl3ČÜŅŗ |
| ¢Ü | 10mL5% H2O2ČÜŅŗ+ÉŁĮæHClČÜŅŗ | 1mL0.1mol•L-1FeCl3ČÜŅŗ |
| ¢Ż | 10mL5% H2O2ČÜŅŗ+ÉŁĮæNaOHČÜŅŗ | 1mL0.1mol•L-1FeCl3ČÜŅŗ |
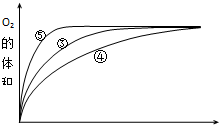
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ķʬ”¢ŹÆÄ«°ō£¬ŅŅ“¼ | B£® | Ķʬ”¢ŹÆÄ«°ō£¬ĻõĖįŅųČÜŅŗ | ||
| C£® | Šæʬ”¢Ķʬ£¬Ļ”ŃĪĖį | D£® | Ķʬ”¢²¬Ę¬£¬FeCl3ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com