
£Ø12·Ö£©ĻĀĶ¼ĪŖ֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļŅŃĀŌČ„£©”£ŅŃÖŖ£ŗA”¢C”¢DŹĒ³£¼ūµÄĘųĢåµ„ÖŹ£¬FĘųĢ弫Ņ×ČÜÓŚĖ®£¬ĒŅŅŗĢ¬³£×öÖĘĄä¼Į”£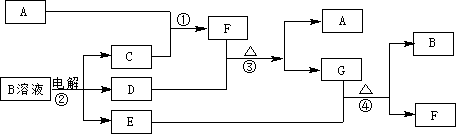
£Ø1£©Š“³ö»ÆѧŹ½A £¬D £¬F £¬GŹōÓŚ ¾§Ģ壻
£Ø2£©¼ų¶ØGÖŠŃōĄė×ӵďµŃé·½·ØŗĶĻÖĻó_____________________________£»
£Ø3£©Dµ„ÖŹŗĶEČÜŅŗ·“Ó¦£¬Éś³ÉŅ»ÖÖ³£¼ūµÄĻū¶¾¼ĮŗĶĘÆ°×¼ĮµÄÓŠŠ§³É·Ö£¬Š“³öD+EČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŗĶ¢ŪµÄ»Æѧ·½³ĢŹ½ ”¢
ӣ
£Ø4£©³£ĪĀĻĀ£¬µē½āBČÜŅŗÖʵĆpH = 12µÄÉÕ¼īČÜŅŗ1000mLŌņ·“Ó¦ÖŠ×ŖŅʵĵē×ÓŹżÄæĪŖ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
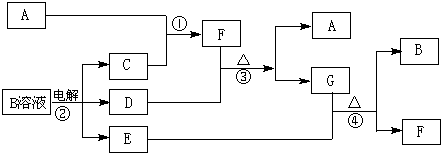
£Ø12·Ö£©ĻĀĶ¼ĪŖ֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļŅŃĀŌČ„£©”£ŅŃÖŖ£ŗA”¢C”¢DŹĒ³£¼ūµÄĘųĢåµ„ÖŹ£¬FĘųĢ弫Ņ×ČÜÓŚĖ®£¬ĒŅŅŗĢ¬³£×öÖĘĄä¼Į”£
£Ø1£©Š“³ö»ÆѧŹ½A £¬D £¬F £¬GŹōÓŚ ¾§Ģ壻
£Ø2£©¼ų¶ØGÖŠŃōĄė×ӵďµŃé·½·ØŗĶĻÖĻó_____________________________£»
£Ø3£©Dµ„ÖŹŗĶEČÜŅŗ·“Ó¦£¬Éś³ÉŅ»ÖÖ³£¼ūµÄĻū¶¾¼ĮŗĶĘÆ°×¼ĮµÄÓŠŠ§³É·Ö£¬Š“³öD+EČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŗĶ¢ŪµÄ»Æѧ·½³ĢŹ½ ”¢
ӣ
£Ø4£©³£ĪĀĻĀ£¬µē½āBČÜŅŗÖʵĆpH = 12µÄÉÕ¼īČÜŅŗ1000mLŌņ·“Ó¦ÖŠ×ŖŅʵĵē×ÓŹżÄæĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗžÄĻŹ”ĮłŠ£øßČżµŚ¶ž“ĪĮŖæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©ĻĀĶ¼ĪŖ֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ”£ŅŃÖŖ£ŗ£Ø1£©A”¢C”¢DŹĒ³£¼ūµÄĘųĢåµ„ÖŹ£¬£Ø2£©FĘųĢ弫Ņ×ČÜÓŚĖ®£¬ĒŅŅŗĢ¬³£ÓĆ×öÖĘĄä¼Į”££Ø3£©GĪŖŅ×ČÜŠŌµÄŃĪ£¬EĪŖ°×É«³Įµķ”££Ø4£©HĪŖĪŽÉ«ŅŗĢå»ÆŗĻĪļ”£
|
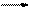
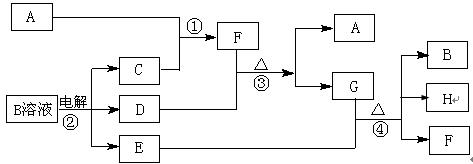 £Ø1£©Š“³öGµÄµē×ÓŹ½ £¬
£Ø1£©Š“³öGµÄµē×ÓŹ½ £¬²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗž±±Ź”Š¢øŠøßÖŠøßČż9ŌĀµ÷ŃŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĶ¼ĪŖ֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļŅŃĀŌČ„£©”£ŅŃÖŖ£ŗA”¢DŹĒ½šŹōµ„ÖŹ£¬LĪŖŗģŗÖÉ«³Įµķ£¬EĪŖŹ³ŃĪµÄÖ÷ŅŖ³É·Ö£¬IµÄĖ®ČÜŅŗ³ŹĒæĖįŠŌ”£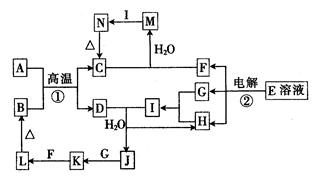
£Ø1£©KµÄ»ÆѧŹ½ĪŖ______________________________”£
£Ø2£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½£ŗ______________________________________”£
£Ø3£©Š“³ö·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½£ŗ______________________________________”£
£Ø4£©ĻņMČÜŅŗÖŠ¼ÓČė×ćĮæµÄIČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________”£
£Ø5£©Š“³öŅ»øöÓÉ»ÆŗĻ·“Ӧɜ³ÉLµÄ»Æѧ·½³ĢŹ½______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģøŹĖąŹ”ŗÓĪ÷ĪåŹŠ²æ·ÖĘÕĶØøßÖŠøßČżµŚŅ»“ĪĮŖŗĻæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĶʶĻĢā
ĻĀĶ¼ĪŖ֊ѧ»Æѧ֊¼øÖÖ³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļŅŃĀŌČ„£©”£ŅŃÖŖ£ŗA”¢DŹĒ½šŹōµ„ÖŹ£¬LĪŖŗģŗÖÉ«³Įµķ£¬EĪŖŹ³ŃĪµÄÖ÷ŅŖ³É·Ö£¬IµÄĖ®ČÜŅŗ³ŹĒæĖįŠŌ”£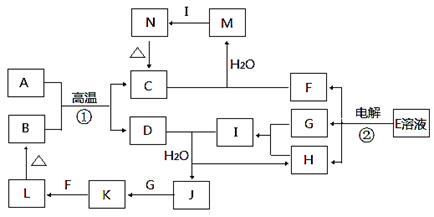
£Ø1£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½£ŗ______________________________________”£
£Ø2£©Š“³ö·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½£ŗ______________________________________”£
£Ø3£©ĻņMČÜŅŗÖŠ¼ÓČė×ćĮæµÄIČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________”£
£Ø4£©Š“³öŅ»øöÓÉ»ÆŗĻ·“Ӧɜ³ÉLµÄ»Æѧ·½³ĢŹ½______________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com