
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά�� �ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ�
���ֺ���������ˮ�е��ܽ������±���ʾ��

�ù��յ���Ҫ�������£�
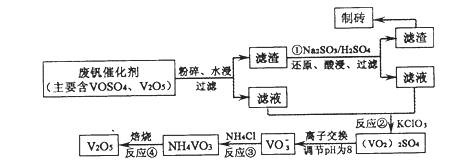
��ش��������⡣
��1����ҵ����V2O5ұ���������������ȼ������û�ѧ����ʽ��ʾΪ ��
��2����Ӧ�ٵ�Ŀ���� ��
��3���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽ�� ��
��4������֪Ũ�ȵ������ữ��H2C2O4��Һ���ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к�������VO2++H2C2O4+H+��VO2++CO2+X��XΪ ��д��ѧʽ����
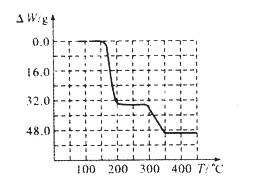
��5���������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯 ����������ͼ��ʾ����NH4VO3�ڷֽ������ ������ţ���
A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3
B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O
C��ͬʱ�ֽ�ʧȥH2O��NH3
D��ͬʱ�ֽ�ʧȥH2��N2��H2O
��֪ʶ�㡿���������� A4 B1 C2
���𰸽�������1��3V2O5��10Al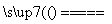 6V��5Al2O3��3�֣�
6V��5Al2O3��3�֣�
��2����V2O5ת��Ϊ�����Ե�VOSO4��3�֣�
��3��NH ��VO
��VO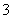 ===NH4VO3����3�֣�
===NH4VO3����3�֣�
��4��H2O��3�֣�
��5��B��3�֣�
������ ����V2O5ұ���������������ȼ�����������V2O5��Ӧ����V����3V2O5��10Al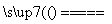 6V��5Al2O3
6V��5Al2O3
�Ƹ������̺����������顢ˮ�������˵õ�����Һ�ͷ�Ӧ�ٵ���ҺΪ��Ӧ�ڵķ�Ӧ���˷�Ӧ�ٵ���Һ��VOSO4������Ӧ�ٵ�Ŀ���Ǽ���������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4��
�Ǹ���NH4VO3������ˮ�����ø��ֽⷴӦ����VO3-����ӦΪ��NH4++VO3-=NH4VO3��
�ȸ��ݵ�ʧ������ȿ�д�������ӷ���Ϊ2VO2++H2C2O4+2H+=2VO2++2CO2+2H2O ��
����NH4VO3���շֽ�ų�������ˮ����ӦΪ
2NH4VO3═V2O5+2NH3��+H2O
234g 34g 18g���ų��İ���������ˮ��������Ϊ34��18����ͼ��2�ι���ļ���ֵ�Ƚӽ�����ʼΪ0��32.0g��һ�㣬���ߵ�200��ʱ���߿�ʼƽֱ����ԼΪ300��ʱ�ֿ�ʼ���٣���350��ʱ����Լ48-32=16gʱ�Ͳ��ٱ仯��������NH4VO3�ڱ��չ������ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O��
��˼·�㲦����������Ϊ�����������ر仯���������ʿ��ܷ����ķ�Ӧ��2NH4VO3═V2O5+2NH3��+H2O�������ʧȥ���������ų��İ���������ˮ��������Ϊ34��18����ͼ�������ı仯����ʼΪ0��32.0g��һ�㣬������Լ16g���Ƿ��Ǻϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4���ƵĻ�����W��X��Y��Z������֮��������¹�ϵ��
��W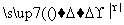 X��H2O��CO2������Z��CO2�D��X��O2
X��H2O��CO2������Z��CO2�D��X��O2
��Z��H2O�D��Y��O2������X��Ca(OH)2�D��Y��CaCO3��
�Իش��������⣺
(1)W��X��Y��Z�Ļ�ѧʽ�ֱ��ǣ�
W��________��X��________��Y��________��Z��________��
(2)����4����ѧ��Ӧ������������ԭ��Ӧ����________(�Ӧ���)����Ӧ����������________(д��ѧʽ)����ԭ����________(д��ѧʽ)��
(3)���ܷ�Ӧ����Һ�н��У�д�������ӷ���ʽ�Լ����ø����ӷ���ʽ��ʾ����һ����ѧ��Ӧ�Ļ�ѧ����ʽ��
���ӷ���ʽ��____________________________________________________________��
��ѧ����ʽ��___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д����CH2ClCH2CH2CH2OHΪԭ���Ʊ�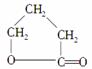 �ĸ�����Ӧ����ʽ(��Ҫ�����Լ���ѡ)��
�ĸ�����Ӧ����ʽ(��Ҫ�����Լ���ѡ)��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________
________________________________________________________________________��
��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӿɿ������ɡ�CH3����CH2����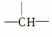 ��
��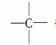 �Ƚṹ��ɵġ����ij����������ͬʱ������4�ֽṹ������̼ԭ���������٣�������������Ӧ��____________��̼ԭ�ӣ���ṹ��ʽ����Ϊ________________________��__________________��
�Ƚṹ��ɵġ����ij����������ͬʱ������4�ֽṹ������̼ԭ���������٣�������������Ӧ��____________��̼ԭ�ӣ���ṹ��ʽ����Ϊ________________________��__________________��
____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl-��S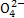 ��Br-��C
��Br-��C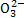 ��HC
��HC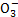 �����ӣ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤��������ͼ��ʾ��
�����ӣ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤��������ͼ��ʾ��
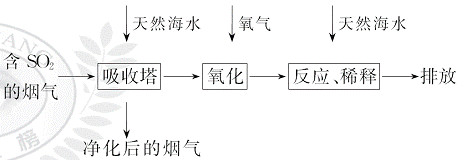
����˵��������ǣ� ��
A.��Ȼ��ˮpH��8��ԭ���Ǻ�ˮ�е�C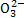 ��HC
��HC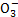 ˮ��
ˮ��
B.��������������������HS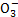 ��S
��S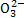 ����������S
����������S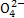
C.����Ӧ��ϡ�͡�ʱ����Ȼ��ˮ��Ŀ�����к͡�ϡ�;�����������ˮ�����ɵ���
D.���ŷš������ĺ�ˮ��S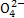 �����ʵ���Ũ�����������������Ȼ��ˮ��ͬ
�����ʵ���Ũ�����������������Ȼ��ˮ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A����CH3COOH�ܽ�CaCO3��CaCO3+2H+=Ca2++H2O+C02��
B��Fe2��SO4��3��Ba��OH��2��Һ��ϣ�Fe3++SO42-+Ba2++3OH��=Fe��OH��3��+ BaSO4��
C�����Ṥҵβ���е�SO2�ù����İ�ˮ���գ�2NH3·H2O+ SO2 =2NH4++ SO32-+H2O
D��Cu����ŨHNO3��Cu+ 4H++ 2NO3һ=Cu2++ 2NO��+ 4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A. ������NaOH��Һ��Al+2OH—=AlO2—+H2��
B. ͭ����ϡ���3Cu+ 8H+ +2NO3—=3Cu2+ +2NO�� + 4H2O
C. ̼��þ�еμ�ϡ���CO32—+2H+ =CO2�� + H2O
D. ϡ�����еμ�����������Һ��H++ OH—=H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�ʾ��ѧ��Ӧ�����ӷ���ʽ��������ȷ����
A��NaAlO2��Һ�еμӹ������AlO2��+H2O+H��= AI(OH)3
B������ͨ����ˮ�У�Cl2+H2O 2H��+Cl�� +ClO��
2H��+Cl�� +ClO��
C����������Һ��ͨ��������CO2��2C6H5O��+CO2+H2O =2C6H5OH +CO32��
D��FeCl3��Һ��Cu��Ӧ��2Fe3++Cu = 2Fe2++ Cu2+
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com