
��ͼװ�â���һ�ֿɳ���أ�װ�â�Ϊ���ء�װ�â�����ӽ���Ĥֻ����Na��ͨ������֪��س�ŵ�Ļ�ѧ����ʽΪ��2Na2S2��NaBr3 Na2S4��3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ���� (����)��
Na2S4��3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ���� (����)��
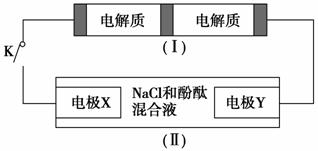
A���պϿ���Kʱ�������Ӵ��ҵ���ͨ�����ӽ���Ĥ
B���պϿ���Kʱ��������ӦʽΪ��3NaBr��2e��===NaBr3��2Na��
C���պϿ���Kʱ��X�缫��ӦʽΪ��2Cl����2e��===Cl2��
D���պϿ���Kʱ������0.1 mol Na��ͨ�����ӽ���Ĥʱ��X�缫�Ϸų���״
��������1.12 L
�����������պϿ���Kʱ��X�� ��������Һ��족˵��X��������YΪ���������ص�����Ǹ�������ԭ������������������ƶ�����A����NaBr3�D��3NaBr����Ԫ�صĻ��ϼ۽��ͣ��ǵõ����Ӷ�����ʧȥ����B����X���������������ĵ缫��ӦʽΪ��2H����2e��===H2������C����������0.1 mol Na��ͨ�����ӽ���Ĥʱ��˵��ת����0.1 mol���ӣ���X�缫����0.05 mol H2���ڱ�״�������Ϊ1.12 L����D��ȷ��
��������Һ��족˵��X��������YΪ���������ص�����Ǹ�������ԭ������������������ƶ�����A����NaBr3�D��3NaBr����Ԫ�صĻ��ϼ۽��ͣ��ǵõ����Ӷ�����ʧȥ����B����X���������������ĵ缫��ӦʽΪ��2H����2e��===H2������C����������0.1 mol Na��ͨ�����ӽ���Ĥʱ��˵��ת����0.1 mol���ӣ���X�缫����0.05 mol H2���ڱ�״�������Ϊ1.12 L����D��ȷ��
�𰸡�D



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ش���������
��1����2FeBr2+3Cl2=2FeCl3+2Br2�ķ�Ӧ��,��������Ԫ����_____________
��2�������������б���ʴ���غ�ɫ����FeCl3���������������ɵ���ɫ��Һ��FeCl2����Ũ�����еμ�KMnO4��Һ��������ɫ���壨Cl2����Cl2��Fe3+��MnO4����������ǿ������˳����____________________��
��3����֪CuO���������ԣ��ܹ��Ͱ�����Ӧ�������������ֵ��ʣ���д���ڼ���������CuO��NH3��Ӧ�Ļ�ѧ����ʽ___________________________
��4��ʵ�����е�Na2SiO3��Һ���ڷ��ã�ƿ����ְ�ɫ�������γɳ��������ӷ���ʽ�� ��ȡƿ�е��ϲ���Һ����ϡ���ᣬ�����������г������ɣ������ӷ���ʽΪ ��
(5)��������ŷ�NOx���ܵ��µĻ���������____________________(������)
��֪����NaOH��Һ����ȫ����NO2�������ֵ��ĺ������Σ���д���÷�Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ķ�Ӧ�У�����
A. �������� B. �ǻ�ԭ�� C. ����������Ҳ���ǻ�ԭ�� D. �������������ǻ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±������ڱ��е�һ���֣�����A��J�����ڱ��е�λ�ã�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | A | D | E | H | ||||
| 3 | B | C | F | G | J |
�ش��������⣺
(1) ��ԭ����ǿ�ĵ����� ���ѧʽ����
(2) D������ͬ��������������� �� ��
(3) F��G��J����Ԫ�ص�ԭ�ӵõ���������ǿ����������˳��Ϊ > >
����Ԫ�ط��ţ���
(4) ��E��F��G��H��J����Ԫ�ص���̬�⻯���У����ȶ����⻯����
���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йظ�����ʴ�������˵����ȷ����(����)
A���ֹ����Դ�������ӣ��ֹܿɱ�����
B��������Ũ�������ۻ����ɱ����ڲ�������ʴ
C���ֹ� ��ͭ��¶��ѷ���һ��ʱ���ֹܲ��ױ���ʴ
��ͭ��¶��ѷ���һ��ʱ���ֹܲ��ױ���ʴ
D�������������ⸯʴʱ��������Ӧ��Fe��3e��===Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧϰ��ѧ����Ҫ���ߣ�����������ʾ���ʱ仯�Ļ�ѧ�����У���ȷ���� (����)��
A���ö��Ե缫��ⱥ��ʳ��ˮʱ�������ĵ缫��ӦʽΪ2Cl����2e��===Cl2��
B������ȼ�ϵ�صĸ�����Ӧʽ��O2��2H2O��4e��===4OH��
C����ͭ����ʱ�����Դ�����������Ǵ�ͭ��������ӦʽΪCu��2e��===Cu2��D�����������绯ѧ��ʴ��������Ӧʽ��Fe��2e��===Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��һ���ò�˿���缫�����ϡ��MgSO4��Һ��װ�ã����Һ�м������Ժ�ָʾ������ʱ��Һ�ʺ�ɫ��(ָʾ����pH��ɫ��Χ��6.8��8.0����ɫ����ɫ����ɫ����ɫ)�ش��������⣺
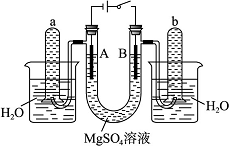
(1)���й��ڵ������е缫������Һ��ɫ�仯���� ����ȷ����______(����)��
����ȷ����______(����)��
��A����Һ�ɺ���
��B����Һ�ɺ���
��A����Һ����ɫ
��B����Һ����ɫ
(2)д�� A���з�����Ӧ�ķ�Ӧʽ��____________________________________________�� (3)д
A���з�����Ӧ�ķ�Ӧʽ��____________________________________________�� (3)д ��B���з�����Ӧ�ķ�Ӧʽ��____________________________________________��
��B���з�����Ӧ�ķ�Ӧʽ��____________________________________________��
(4)����a��������ķ�����_ __________________________________________________��
(5)����b��������ķ�����_________________________________________________��
(6)���һ��ʱ����жϵ�Դ�������Һ�����ձ��ڹ۲쵽��������________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӵļ۵����Ų��У���Ӧ�ڵ�һ�����������ǣ� ����
A. 3s23p1 B. 3s23p2 C. 3s23p3 D. 3s23p4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����һ����Ҫ�ĵ��Ļ������NA��ʾ�����ӵ�������ֵ������������ȷ���� (����)��
A��1 mol NH4�����еĵ�����Ϊ11NA
B��NH4����NԪ�صĻ��ϼ�Ϊ��3����������ԭ��Ӧ�г���������
C��0.1 L 3 mol��L��1��NH4NO3��Һ�е�ԭ��������0.6NA
D��������мȺ������Ӽ����ֺ��й��ۼ������Ȼ����ֻ�������Ӽ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com