
”¾ĢāÄæ”æ¶ĢÖÜĘŚÖ÷×åŌŖĖŲA”¢B”¢C”¢DµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢CĶ¬Ö÷×壬B”¢C”¢DĶ¬ÖÜĘŚ£¬AŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹżµÄ3±¶£¬BŹĒ¶ĢÖÜĘŚŌŖĖŲÖŠŌ×Ó°ė¾¶×ī“óµÄÖ÷×åŌŖĖŲ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄŌŖĖŲ·ūŗÅ___________£»BµÄŌŖĖŲĆū³Ę___________£»
£Ø2£©DµÄŌ×ӵĵē×ÓŹ½____________£»CŌ×ӵĵē×ÓÅŲ¼Ź½___________________”£
£Ø3£©A”¢B”¢CČżÖÖŌŖĖŲŠĪ³ÉµÄ¼ņµ„Ąė×ӵİė¾¶Óɓ󵽊”µÄĖ³ŠņŹĒ_____________”£
£Ø4£©CA2ÓėDŌŖĖŲµÄµ„ÖŹŌŚĖ®ČÜŅŗÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______________________”£
”¾“š°ø”æ O ÄĘ ĀŌ 1s22s22p6 3s23p4 S2-”¢O2-”¢Na+ SO2 + Cl2 + 2H2O == H2SO4 + 2HCl
”¾½āĪö”æŹŌĢā·ÖĪö£ŗ¶ĢÖÜĘŚÖ÷×åŌŖĖŲA”¢B”¢C”¢DµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹżµÄ3±¶£¬ŌņAĪŖOŌŖĖŲ£»A”¢CĶ¬Ö÷×壬CĪŖSŌŖĖŲ,DĪŖClŌŖĖŲ£»B”¢C”¢DĶ¬ÖÜĘŚ£¬BŹĒ¶ĢÖÜĘŚŌŖĖŲÖŠŌ×Ó°ė¾¶×ī“óµÄÖ÷×åŌŖĖŲ£¬ŌņBĪŖNaŌŖĖŲ£»¹ŹDµÄŌ×ӵĵē×ÓŹ½ĪŖ£ŗ![]() £¬CŌ×ӵĵē×ÓÅŲ¼Ź½1s22s22p63s23p4£¬A”¢B”¢CČżÖÖŌŖĖŲŠĪ³ÉµÄ¼ņµ„Ąė×ÓO2”¢Na+”¢S2-£¬ČżÖÖĪ¢Į£ÖŠS2-µē×Ó²ćŹż×ī¶ą£¬¹ŹS2-°ė¾¶×ī“ó£¬O2”¢Na+µē×Ó²ćŹżĻąĶ¬£¬µ«O2-µÄŗĖµēŗÉŹżŠ”£¬Ōņ°ė¾¶“󣬣¬¹Ź°ė¾¶Óɓ󵽊”Ė³ŠņĪŖ£ŗS2->O2->Na+£»CA2ÓėDŌŖĖŲµÄµ„ÖŹŌŚĖ®ČÜŅŗÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒSO2 + Cl2 + 2H2O == H2SO4 + 2HCl”£
£¬CŌ×ӵĵē×ÓÅŲ¼Ź½1s22s22p63s23p4£¬A”¢B”¢CČżÖÖŌŖĖŲŠĪ³ÉµÄ¼ņµ„Ąė×ÓO2”¢Na+”¢S2-£¬ČżÖÖĪ¢Į£ÖŠS2-µē×Ó²ćŹż×ī¶ą£¬¹ŹS2-°ė¾¶×ī“ó£¬O2”¢Na+µē×Ó²ćŹżĻąĶ¬£¬µ«O2-µÄŗĖµēŗÉŹżŠ”£¬Ōņ°ė¾¶“󣬣¬¹Ź°ė¾¶Óɓ󵽊”Ė³ŠņĪŖ£ŗS2->O2->Na+£»CA2ÓėDŌŖĖŲµÄµ„ÖŹŌŚĖ®ČÜŅŗÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒSO2 + Cl2 + 2H2O == H2SO4 + 2HCl”£


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æI.»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖ»ł“”µÄѧæĘ£¬»ÆѧĖłČ”µĆµÄ·įĖ¶³É¹ūÓėŹµŃéµÄÖŲŅŖ×÷ÓĆ·Ö²»æŖ”£½įŗĻĻĀĮŠŹµŃé×°ÖĆĶ¼»Ų“šĪŹĢā£ŗ

£Ø1£©Š“³öÉĻŹöĶ¼ÖŠŅĒĘ÷µÄĆū³Ę£ŗ¢Ł_______£»¢Ś____”£
£Ø2£©ČōĄūÓĆ×°ÖĆI·ÖĄėŅŅĖį£Ø·Šµć118”ę£©ŗĶŅŅĖįŅŅõ„£Ø·Šµć77.1”ę£©µÄ»ģŗĻĪļ£¬»¹Č±ÉŁµÄ²£Į§ŅĒĘ÷ÓŠ________£¬½«ŅĒĘ÷²¹³äĶźÕūŗó½ųŠŠµÄŹµŃé²Ł×÷µÄĆū³ĘĪŖ_______£»ŹµŃ鏱ŅĒĘ÷¢ŚÖŠĄäČ“Ė®µÄ½ųæŚĪŖ____£ØĢī”°f”±»ņ ”°g”±£©£¬ÕōĮóÉÕĘæÄŚĖé“ÉʬµÄ×÷ÓĆŹĒ________”£
£Ø3£©ĻÖŠčÅäÖĘ250 mL 0.1 moI.L-1NaClČÜŅŗ£¬×°ÖĆIIŹĒijĶ¬Ń§×ŖŅĘČÜŅŗµÄŹ¾ŅāĶ¼£¬Ķ¼ÖŠÓŠĮ½“¦“ķĪó·Ö±šŹĒ________£¬________”£
II. ¢Łŗ£“ųµČŌåĄąĪļÖŹ¾¹ż“¦Ąķŗó£¬æÉŅŌµĆµ½µāĖ®£¬ĻņµāĖ®ÖŠ¼ÓČėĖÄĀČ»ÆĢ¼ŅŌĢįČ”µāµ„ÖŹµÄŹµŃé²Ł×÷½Š________£¬øĆ²Ł×÷ŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠ________”£
¢ŚÄ³NaClѳʷ֊æÉÄÜŗ¬ÓŠSO42-£¬CO32-£¬ĪŖ¼ģŃéŌÓÖŹĄė×ӵēęŌŚ£¬²ÉČ”ČēĻĀŹµŃé²½Öč£ŗ
ѳʷ![]() ĪŽĆ÷ĻŌĻÖĻó
ĪŽĆ÷ĻŌĻÖĻó![]() ĪŽĆ÷ĻŌĻÖĻó”£
ĪŽĆ÷ĻŌĻÖĻó”£
Ōņ¼ÓČėµÄŹŌ¼ĮAĪŖ_______£¬BĪŖ_______£¬øĆĻÖĻóÖ¤Ć÷ѳʷ֊²»ŗ¬ÓŠ____”£
¢ŪŌŚŗóŠųŹµŃéÖŠŠčŅŖŹ¹ÓĆ450mL0.5 mol”¤L- 1NaClČÜŅŗ£¬ĪŖÅäÖĘøĆÅضČNaClČÜŅŗ½ųŠŠŹµŃ飬ŠčÓĆĶŠÅĢĢģĘ½³ĘČ”NaCl_______g”£ÅäÖĘNaClČÜŅŗŹ±£¬Čō³öĻÖĻĀĮŠ²Ł×÷£¬»įŹ¹ÅäÖĘÅضČĘ«øߵďĒ____________
A£®ĢģĘ½ķĄĀė¼ŗŠāŹ“ B£®ÅäÖĘ¹ż³ĢÖŠĪ“ÓĆÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō
C£®×ŖŅĘČÜŅŗŹ±ÓŠČÜŅŗ½¦³ö D£®¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ(1)98%µÄÅØH2SO4(¦Ń=1.84g/ mL)µÄÅضČĪŖ______mol/L£¬ÓĆøĆÅØĮņĖįÅäÖĘ500ml0.5mol/LµÄĻ”H2SO4£¬ŠčŅŖĮæČ”ÅØH2SO4µÄĢå»żĪŖ_______mL(Š”Źżµćŗó±£ĮōŅ»Ī»ÓŠŠ§Źż×Ö)”£
(2)Čē¹ūŹµŃéŹŅÓŠ10mL”¢20mL”¢50mLĮæĶ²£¬Ó¦Ń”ÓĆ______mLĮæĶ²£¬ŹµŃéÖŠ»¹ŠčŅŖÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢_________________________”£
(3)Ź¹ÓĆČŻĮæĘæĒ°±ŲŠė½ųŠŠµÄŅ»²½²Ł×÷ŹĒ_____________________”£
(4)ÅäÖĘŹ±,Ņ»°ć·ÖĪŖŅŌĻĀ¼øøö²½Öč:
¢ŁĮæČ” ¢Ś¼ĘĖć ¢ŪČܽā ¢ÜŅ”ŌČ ¢Ż×ŖŅĘ ¢ŽĻ“µÓ ¢ß¶ØČŻ ¢ąĄäČ“¢įŅ”¶Æ
ĘäÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ________________________”£
A. ¢Ś¢Ł¢Ū¢ą¢Ż¢į¢ß¢Ž¢Ü B. ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢į¢ß¢Ü C. ¢Ś¢Ł¢Ū¢Ż¢Ž¢ß¢Ü¢ą¢į
(5) ČōŹµŃéÖŠ³öĻÖĻĀĮŠĻÖĻó£¬Ōģ³ÉĖłÅäČÜŅŗÅضČĘ«øßµÄÓŠ______
A.ÅØĮņĖįĻ”ŹĶŗóĪ“ĄäÖĮŹŅĪĀ¼“×ŖŅĘÖĮČŻĮæĘæ½ųŠŠ¶ØČŻ
B.¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß
C.ĮæČ”ŗĆÅØĮņĖįµ¹ČėÉÕ±Čܽāŗó£¬ÓĆĖ®Ļ“µÓĮæĶ²2-3“Ī£¬½«Ļ“µÓŅŗµ¹ČėÉÕ±
D”¢ŅĘŅŗŗóÉÕ±Ī“Ļ“µÓ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠø÷×éÖø¶ØµÄŌŖĖŲ£¬²»ÄÜŠĪ³ÉAB2ŠĶ»ÆŗĻĪļµÄŹĒ£Ø £©
A.2s22p2 ŗĶ2s22p4 B. 3s23p4 ŗĶ2s22p4 C.3s2ŗĶ2s22p5 D. 3s1ŗĶ3s23p5
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A. Ē¦Šīµē³Ų·ÅµēŹ±øŗ¼«ÖŹĮæ¼õĒį£¬³äµēŹ±Ńō¼«ÖŹĮæŌö¼Ó
B. Ķ¬ĪĀĻĀ£¬0.1 mol”¤L£1“×ĖįČÜŅŗpH£½a£¬0.01 mol”¤L£1“×ĖįČÜŅŗpH£½b£¬Ōņa£«1<b
C. øÖĢśĖ®Õ¢æÉÓĆĪžÉüŃō¼«µÄŅõ¼«±£»¤·Ø»ņĶā¼ÓµēĮ÷µÄŅõ¼«±£»¤·Ø½ųŠŠ·Ą»¤
D. Ņ»¶ØĢõ¼žĻĀ·“Ó¦N2£«3H2![]() 2NH3£¬µ±3vÕż(H2)£½2vÄę(NH3)£¬Ōņ·“Ó¦“ļµ½Ę½ŗā
2NH3£¬µ±3vÕż(H2)£½2vÄę(NH3)£¬Ōņ·“Ó¦“ļµ½Ę½ŗā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”湤ŅµÉĻ³£ÓĆ·°ĀÆŌü(Ö÷ŅŖŗ¬FeO”¤V2O5£¬»¹ÓŠÉŁĮæSiO2”¢P2O5µČŌÓÖŹ)ĢįČ”V2O5µÄĮ÷³ĢČēĻĀ£ŗ

£Ø1£©±ŗÉÕµÄÄæµÄŹĒ½«FeO”¤V2O3×Ŗ»ÆĪŖæÉČÜŠŌNaVO3£¬øĆ¹ż³ĢÖŠ±»Ńõ»ÆµÄŌŖĖŲŹĒ_______________£»½ž³öŌüµÄÖ÷ŅŖ³É·ÖĪŖ____________________(Ģī»ÆѧŹ½)”£
£Ø2£©ÓĆMgSO4ČÜŅŗ³ż¹č”¢Į׏±£¬ĀĖŌüµÄÖ÷ŅŖ³É·ÖĪŖ__________”£
£Ø3£©ŌŚ±ŗÉÕNH4VO3µÄ¹ż³ĢÖŠ£¬¹ĢĢåÖŹĮæµÄ¼õÉŁÖµ(ׯ×ų±ź)ĖęĪĀ¶Č±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£¬210”ꏱ£¬Ź£Óą¹ĢĢåĪļÖŹµÄ»ÆѧŹ½ĪŖ_____________________”£

£Ø4£©ÓÉV2O5Ņ±Į¶½šŹō·°²ÉÓĆĀĮČČ·Ø£¬Ņż·¢ĀĮČČ·“Ó¦µÄŹµŃé²Ł×÷ŹĒ__________________.
£Ø5£©½«V2O5ČÜÓŚ×ćĮæĻ”ĮņĖįµĆµ½250mL(VO2)2SO4ČÜŅŗ”£Č”25.00mLøĆČÜŅŗӌ׶ŠĪĘæÖŠ£¬ÓĆ0.1000 mol”¤L-1H2C2O4±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±Ļūŗıź×¼ČÜŅŗµÄĢå»żĪŖ20.00mL”£ŅŃÖŖµĪ¶Ø¹ż³ĢÖŠH2C2O4±»Ńõ»ÆĪŖCO2£¬VO2+(»ĘÉ«)±»»¹ŌĪŖVO2+(Ą¶É«)”£
¢ŁøƵĪ¶ØŹµŃé²»ŠčŅŖĮķĶā¼ÓČėÖøŹ¾¼Į£¬“ļµ½µĪ¶ØÖÕµćµÄĻÖĻóŹĒ___________________”£
¢Ś(VO2)2SO4ČÜŅŗÖŠČÜÖŹµÄĪļÖŹµÄĮæÅضČĪŖ___________________”£
¢Ū“ļµ½µĪ¶ØÖÕµćŹ±£¬ø©ŹÓµĪ¶Ø¹Ü¶ĮŹż½«Ź¹½į¹ū_________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆŅŅČ²ĪŖŌĮĻ£¬ÖĘČ”CH2Br-CHBrCl£¬æÉŠŠµÄ·“Ó¦Ķ¾¾¶ŹĒ£Ø £©
A.ĻČ¼ÓCl2£¬ŌŁ¼ÓBr2B.ĻČ¼ÓCl2£¬ŌŁ¼ÓHBr
C.ĻČ¼ÓHCl£¬ŌŁ¼ÓHBrD.ĻČ¼ÓHCl£¬ŌŁ¼ÓBr2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æøł¾ŻÓŠ»ś»ÆŗĻĪļµÄĆüĆūŌŌņ£¬ĻĀĮŠĆüĆūÕżČ·µÄŹĒ
A. 3-¼×»ł-2-¶”Ļ© B. 2,2-¶ž¼×»ł¶”Ķé
C. 3-Āȶ”Ķé D. 2-ŅŅ»łĪģĶé
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æīę(Ce)ŹĒļēĻµ½šŹōŌŖĖŲ”£æÕĘųĪŪČ¾ĪļNOĶس£ÓĆŗ¬Ce4+µÄČÜŅŗĪüŹÕ£¬Éś³ÉHNO2”¢NO3”„£¬ŌŁĄūÓƵē½ā·Ø½«ÉĻŹöĪüŹÕŅŗÖŠµÄHNO2×Ŗ»ÆĪŖĪŽ¶¾ĪļÖŹ£¬Ķ¬Ź±Éś³ÉCe4+£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
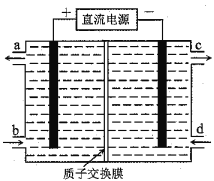
A. H+ÓÉÓŅŹŅ½ųČė×óŹŅ
B. Ce4+“Óµē½ā²ŪµÄcæŚĮ÷³ö£¬ĒŅæÉŃ»·Ź¹ÓĆ
C. Ņõ¼«µÄµē¼«·“Ó¦Ź½£ŗ2HNO2+6H++6e”„=N2”ü+4H2O
D. ČōÓĆ¼×ĶéČ¼ĮĻµē³Ų×÷ĪŖµēŌ“£¬µ±Ļūŗıź×¼×“æöĻĀ33.6L¼×Ķ鏱£¬ĄķĀŪÉĻæÉ×Ŗ»ÆHNO22mol
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com