
| c(��)��V(��) |
| V(��) |
| c(��)��V(��) |
| V(��) |


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��5.6g����������Cl2��Ӧ��ʧȥ�ĵ�����Ϊ0.2NA |
| B�����³�ѹ�£�18g H2O����10NA������ |
| C�������£�1L 0.1mol/L AlCl3��Һ�к�Al3+��Ϊ0.1NA |
| D��1 mol Cu������Ũ���ᷴӦ����2NA��SO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
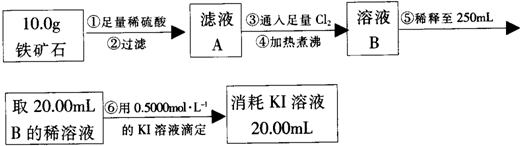

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ζ������У���ƿ������Һ���� |
| B����ƿ������ˮϴ����δ�����T���еζ� |
| C����ʽ�ζ���δ�ñ�������ϴ |
| D���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ���ֹʱ������ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ˮϴ����ʽ�ζ��ܺ�װ���������еζ� |
| B��������ˮϴ����ƿ������NaOH��Һ��ϴ����װ��NaOH��Һ���еζ� |
| C���ü�ʽ�ζ���ȡ10.00 mL NaOH��Һ����������ˮϴ������ƿ�У��ټ�����������ˮ���еζ� |
| D���÷�̪��ָʾ��������ɫ�ձ���ɫʱ��ֹͣ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
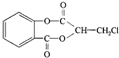 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����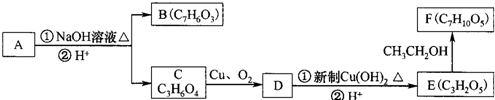
 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹����
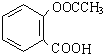
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH | ŨH2SO4 |
| 170�� |
| H2 |
| ����/�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
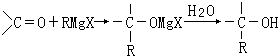
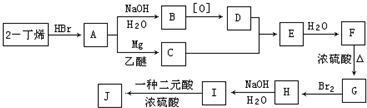
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
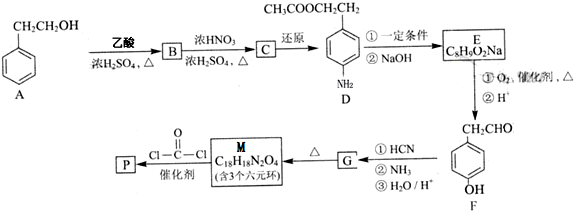
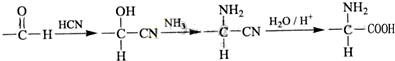
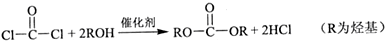
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com