
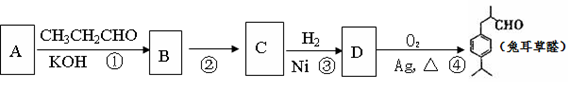
 2CH3CHO
2CH3CHO  CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO 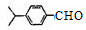 ��2�֣���
��2�֣���  ��2�֣�
��2�֣� +2H2O ��2�֣�
+2H2O ��2�֣�
 ��B�Ľṹ��ʽ��
��B�Ľṹ��ʽ�� ��
�� ����4��A��ͬ���칹���з���������������8�֡�
����4��A��ͬ���칹���з���������������8�֡�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
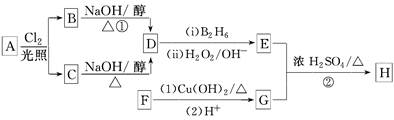
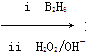 RCH2CH2OH(����B2H6������)
RCH2CH2OH(����B2H6������)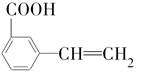 ��
��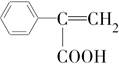 ���������ֵĽṹ��ʽ�ֱ���___________________________________��
���������ֵĽṹ��ʽ�ֱ���___________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
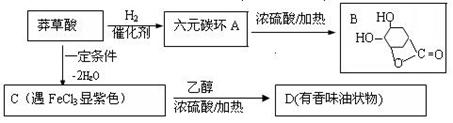
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
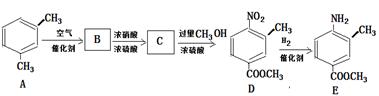
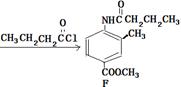
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
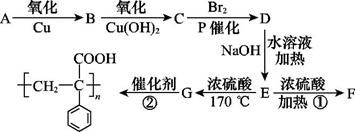
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
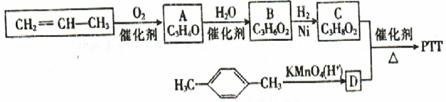
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
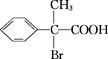
| A�����ɱ�ŵ���ķ�Ӧ����ȡ����Ӧ |
| B��FeCl3��Һ������˾ƥ�ֺ�����Ϣʹ |
| C�������±�ŵ����ˮ�е��ܽ��С������Ϣʹ |
| D����ŵ�������������ʣ�Ҳ�ǰ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
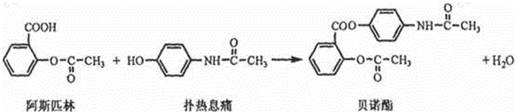
 �辭���������ֺϳ�;��( )��
�辭���������ֺϳ�;��( )��| A����ȥ���ӳɡ���ȥ | B���ӳɡ���ȥ����ˮ |
| C���ӳɡ���ȥ���ӳ� | D��ȡ������ȥ���ӳ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com