
���� ��1����ˮ������ǡ�÷�Ӧ�����Ȼ�泥���Һ�����ԣ��������ԣ���ˮ������
��2��һˮ�ϰ����������Ϊǿ�ᣬ�������Һ��pH��7��˵���������
��3����V1=V2��c1=c2����ˮ������ǡ�÷�Ӧ�����Ȼ�泥�笠�����ˮ�������ԣ�
��4����V1=V2���һ��Һ��pH��7����Һ�����ԣ���Һ�е����ʿ���Ϊ�Ȼ�泥����Ȼ�狀����ᣮ
��� �⣺��1��һˮ�ϰ����������Ϊǿ�ᣬ�����ߵ����ʵ�����Ӧʱ�����Ȼ�泥���Һ�����ԣ��������Һ��pH=7����ˮ����������c1V1 ����c2V2����Һ�д��ڵ���ƽ�⣬��c��H+��+c��NH4+��=c��Cl-��+c��OH-��������ҺpH=7����c��H+��=c��OH-����c��NH4+��=c��Cl-����
�ʴ�Ϊ�����ڣ����ڣ�
��2��һˮ�ϰ����������Ϊǿ�ᣬ�������Һ��pH��7��˵�������������c1V1 ����c2V2����Һ�д��ڵ���ƽ�⣬��c��H+��+c��NH4+��=c��Cl-��+c��OH-��������ҺpH��7����c��H+����c��OH-����c��NH4+����c��Cl-����
�ʴ�Ϊ�����ڣ����ڣ�
��3����V1=V2��c1=c2����ˮ������ǡ�÷�Ӧ�����Ȼ�泥�笠�����ˮ�������ԣ�笠�����Ũ�ȼ�С��������Һ������Ũ�ȵĴ�С��ϵΪc��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��4����V1=V2���һ��Һ��pH��7����Һ�����ԣ���Һ�е����ʿ���Ϊ�Ȼ�泥����Ȼ�狀����ᣬ��c1=c2ʱǡ�÷�Ӧ�����Ȼ�泥��Ȼ��ˮ����Һ�����ԣ����Բ�һ���� c1��c2��
�ʴ�Ϊ����һ����c1=c2ʱ������ǡ�÷�Ӧ����NH4Cl��Һ��pH��7��c1��c2ʱ��pHҲ����С��7��
���� ���⿼���кͷ�Ӧ������ˮ�⡢pHֵ��֪ʶ����Ŀ�Ѷ��еȣ�ע��һˮ�ϰ�Ϊ������ʵ����ʣ�ע����Һ������ԭ������ã������ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϴ����ʽ�ζ��ܺ�����װ���������еζ� | |
| B�� | ��ƿ�ô���NaOH��Һ��ϴ����װ��NaOH ��Һ���еζ� | |
| C�� | ���������У��ζ�ǰƽ�Ӷ������ζ������Ӷ��� | |
| D�� | �÷�̪��ָʾ��������ɫ����ȥ��ֹͣ�ΛV���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
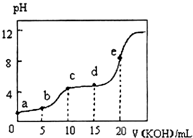 �����£���0.10mol•L-1KOH��Һ�ζ�10.00mL 0.10mol•L-1ij��Ԫ����H2R��Һ�����õζ�������ͼ��ʾ������������ȷ���ǣ�������
�����£���0.10mol•L-1KOH��Һ�ζ�10.00mL 0.10mol•L-1ij��Ԫ����H2R��Һ�����õζ�������ͼ��ʾ������������ȷ���ǣ�������| A�� | a����ʾ��Һ�У�$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$��1012 | |
| B�� | c����ʾ��Һ�У�c��K+����c��HR-����c��H2R����c��R2-�� | |
| C�� | e����ʾ��Һ�У�c��H+��=c��HR-��+2c��H2R��+c��OH-�� | |
| D�� | �������d��ʱc��HR-����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
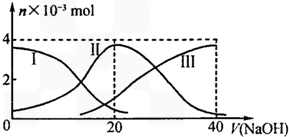 25��ʱ����20mL0.2mol/LH2A��Һ�еμ�0.2mol/LNaOH��Һ���й������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�ж�����˵����ȷ���ǣ�������
25��ʱ����20mL0.2mol/LH2A��Һ�еμ�0.2mol/LNaOH��Һ���й������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�ж�����˵����ȷ���ǣ�������| A�� | �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� | |
| B�� | ��Na2A��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С | |
| C�� | ��ʹNaHA��Һ�����ԣ����������м����� | |
| D�� | ��V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ��[Na+]��[HA-]��[H+]��[A2-]��[OH-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
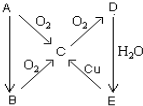 A��B��C��D��E����һ�������µ�ת��������ͼ��ʾ��
A��B��C��D��E����һ�������µ�ת��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�У�K+��MnO4+��SO42-Cl- | |
| B�� | ��ʹ�����Ժ�ɫ����Һ��Fe2+��NO3-��Na+��SO42- | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=l0-12��ˮ��Һ�У�NH4+��Al3+��NO3-��Cl- | |
| D�� | ��������Һ�У�Cu2+��Al3+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com