
ŌŚŌÖŗóÖŲ½ØÖŠ£¬ŅūÓĆĖ®°²Č«Õ¼ÓŠ¼«ĪŖÖŲŅŖµÄµŲĪ»£¬ Ä³ŃŠ¾æŠ”×éĢįȔȿ“¦±»ĪŪČ¾µÄĖ®Ō“½ųŠŠ·ÖĪö£¬²¢øų³öĮĖČēĻĀŹµŃéŠÅĻ¢£ŗĘäÖŠŅ»“¦±»ĪŪČ¾µÄĖ®Ō“ŗ¬ÓŠA”¢BĮ½ÖÖĪļÖŹ£¬Ņ»“¦ŗ¬ÓŠC”¢DĮ½ÖÖĪļÖŹ£¬Ņ»“¦ŗ¬ÓŠEĪļÖŹ£¬A”¢B”¢C”¢D”¢EĪåÖÖ³£¼ū»ÆŗĻĪļ¶¼ŹĒÓÉĻĀ±ķÖŠµÄĄė×ÓŠĪ³ÉµÄ£ŗ
| ŃōĄė×Ó | K£«”¢Na£«”¢Cu2£«”¢Al3£« |
| ŅõĄė×Ó | SO |
ĪŖĮĖ¼ų±šÉĻŹö»ÆŗĻĪļ£¬·Ö±šĶź³ÉŅŌĻĀŹµŃ飬Ęä½į¹ūŹĒ£ŗ
¢Ł½«ĖüĆĒČÜÓŚĖ®ŗó£¬DĪŖĄ¶É«ČÜŅŗ£¬ĘäĖū¾łĪŖĪŽÉ«ČÜŅŗ”£
¢Ś½«EČÜŅŗµĪČėµ½CČÜŅŗÖŠ£¬³öĻÖ°×É«³Įµķ£¬¼ĢŠųµĪ¼Ó³ĮµķČܽā”£
¢Ū½ųŠŠŃęÉ«·“Ó¦ŹµŃ飬ֻӊB”¢Cŗ¬ÓŠ¼ŲĄė×Ó”£
¢ÜŌŚø÷ČÜŅŗÖŠ¼ÓČėBa(NO3)2ČÜŅŗ£¬ŌŁ¼ÓČė¹żĮæĻ”ĻõĖį£¬AÖŠ·Å³öĪŽÉ«ĘųĢ壬C”¢DÖŠ²śÉś°×É«³Įµķ”£
¢Ż½«B”¢DĮ½ČÜŅŗ»ģŗĻ£¬Ī“¼ū³Įµķ»ņĘųĢåÉś³É”£
øł¾ŻÉĻŹöŹµŃéĻÖĻóĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)Š“³öB”¢C”¢DµÄ»ÆѧŹ½£ŗB________”¢C________”¢D________”£
(2)½«ŗ¬1 mol AµÄČÜŅŗÓėŗ¬1 mol EµÄČÜŅŗ·“Ó¦ŗóÕōøÉ£¬½öµĆµ½Ņ»ÖÖ»ÆŗĻĪļ£¬øĆ»ÆŗĻĪļĪŖ________”£
(3)Š“³öŹµŃé¢Ś·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½________________________________________
________________________________________________________________________ӣ
(4)C³£ÓĆ×÷¾»Ė®¼Į£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾Ęä¾»Ė®ŌĄķ_______________________________
________________________________________________________________________ӣ
½āĪö£ŗøł¾ŻĢāøÉæÉÖŖ£¬AŗĶB”¢CŗĶD¾łÄÜ“óĮæ¹²“ę”£ÓÉ¢ŁÖŖ£ŗDČÜŅŗÖŠŗ¬Cu2£«£¬ŌņCÖŠ²»ŗ¬OH£”¢HCO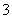 ”£ÓÉ¢ŚÖŖ£ŗEČÜŅŗÖŠŗ¬OH££¬CČÜŅŗÖŠŗ¬Al3£«”£ÓÉ¢ŪÖŖ£ŗB”¢CČÜŅŗÖŠŗ¬ÓŠ¼ŲĄė×Ó£¬A”¢D”¢EÖŠ²»ŗ¬¼ŲĄė×Ó”£ÓÉ¢ÜÖŖ£ŗAÖŠŗ¬HCO
”£ÓÉ¢ŚÖŖ£ŗEČÜŅŗÖŠŗ¬OH££¬CČÜŅŗÖŠŗ¬Al3£«”£ÓÉ¢ŪÖŖ£ŗB”¢CČÜŅŗÖŠŗ¬ÓŠ¼ŲĄė×Ó£¬A”¢D”¢EÖŠ²»ŗ¬¼ŲĄė×Ó”£ÓÉ¢ÜÖŖ£ŗAÖŠŗ¬HCO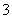 £¬C”¢DČÜŅŗÖŠŗ¬SO
£¬C”¢DČÜŅŗÖŠŗ¬SO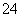 £¬B”¢EÖŠ²»ŗ¬SO
£¬B”¢EÖŠ²»ŗ¬SO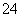 ”£ÓÉ¢ŻæÉÖŖ£ŗBÖŠ²»ŗ¬OH£”¢HCO
”£ÓÉ¢ŻæÉÖŖ£ŗBÖŠ²»ŗ¬OH£”¢HCO ”£×ŪÉĻĖłŹö£¬
”£×ŪÉĻĖłŹö£¬
DĪŖCuSO4 , CĪŖKAl(SO4)2£¬EĪŖNaOH £¬BĪŖKNO3£¬AĪŖNaHCO3”£
“š°ø£ŗ(1)KNO3””KAl(SO4)2””CuSO4””(2)Na2CO3
(3)Al3£«£«3OH£===Al(OH)3”ż£»Al(OH)3£«OH£===AlO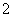 £«2H2O
£«2H2O
(4)Al3£«£«3H2O===Al(OH)3(½ŗĢå)£«3H£«



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
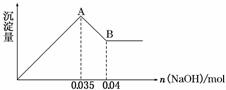
Ä³Ń§ÉśÓĆNaHCO3ŗĶKHCO3×é³ÉµÄ»ģŗĻĪļ½ųŠŠŹµŃ飬²āµĆČēĻĀŹż¾Ż(Ćæ“Ī¼ÓČėµÄŃĪĖįµÄĪļÖŹµÄĮæÅضČĻąµČ)£¬ĻĀĮŠ·ÖĪöÕżČ·µÄŹĒ(””””)
| ŃĪĖį/mL | 50 | 50 | 50 |
| m(»ģŗĻĪļ)/g | 9.2 | 15.7 | 27.6 |
| V(CO2)(±ź×¼×“æö)/L | 2.24 | 3.36 | 3.36 |
A£®ŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ3.0 mol”¤L£1
B£®»ģŗĻĪļÖŠNaHC O3µÄÖŹĮæ·ÖŹżĪŖ54.3%
O3µÄÖŹĮæ·ÖŹżĪŖ54.3%
C£®9.2 g»ģŗĻĪļÖŠKHCO3µÄĪļ ÖŹµÄĮæĪŖ0.05 mol
ÖŹµÄĮæĪŖ0.05 mol
D£®15.7 g»ģŗĻĪļĒ”ŗĆÓėŃĪĖįĶźČ«·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠ»śĪļµÄ±ķŹ¾·½·Ø¶ąÖÖ¶ąŃł£¬ĻĀĆęŹĒ³£ÓƵÄÓŠ»śĪļµÄ±ķŹ¾·½·Ø£ŗ
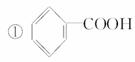 ¢Ś
¢Ś ””¢ŪCH4
””¢ŪCH4
¢Ü ””¢Ż
””¢Ż
¢Ž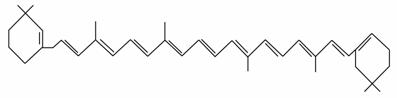
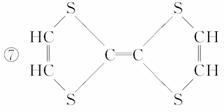
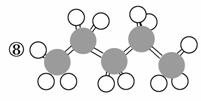
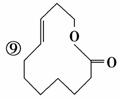
¢ā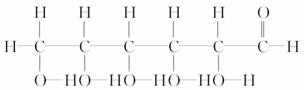
(1)ÉĻŹö±ķŹ¾·½·ØÖŠŹōÓŚ½į¹¹¼ņŹ½µÄĪŖ__________£»
ŹōÓŚ½į¹¹Ź½µÄĪŖ________£»
ŹōÓŚ¼üĻߏ½µÄĪŖ________£»
ŹōÓŚ±ČĄżÄ£ŠĶµÄĪŖ________£»
ŹōÓŚĒņ¹÷Ä£ŠĶµÄĪŖ________”£
(2)Š“³ö¢įµÄ·Ö×ÓŹ½£ŗ________”£
(3)Š“³ö¢āÖŠ¹ŁÄÜĶŵĵē×ÓŹ½£ŗ________”¢________”£
(4)¢ŚµÄ·Ö×ÓŹ½ĪŖ________£¬×ī¼ņŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĀČĖ®ÖŠŗ¬ÓŠ¶ąÖֳɷ֣¬Ņņ¶ų¾ßÓŠ¶ąÖÖŠŌÖŹ£¬øł¾ŻĀČĖ®·Ö±šÓėČēĶ¼ĖÄÖÖĪļÖŹ·¢ÉśµÄ·“Ó¦ĢīæÕ(a”¢b”¢c”¢dÖŲŗĻ²æ·Ö“ś±ķĪļÖŹ¼ä·“Ó¦£¬ĒŅĀČĖ®×ćĮæ)”£
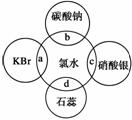
(1)ÄÜÖ¤Ć÷ĀČĖ®¾ßÓŠĘư׊ŌµÄŹĒ________(Ģī”°a”±”¢”°b”±”¢”°c”±»ņ”°d”±)£»
(2)c¹ż³ĢÖŠµÄĻÖĻóŹĒ_____________________________________________________£»
(3)b¹ż³ĢÖŠµÄĄė×Ó·½³ĢŹ½ĪŖ_______________________________________________£»
(4)a¹ż³ĢÖŠµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃ¼ģ²ā³öpH£½1µÄijĪ“ÖŖČÜŅŗÖŠŗ¬ÓŠAl3£«ŗĶNO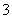 £¬Čō¼ģŃé“ĖČÜŅŗÖŠŹĒ·ń“óĮæ“ęŌŚŅŌĻĀ6ÖÖĄė×Ó£ŗ¢ŁClO£ ¢ŚNH
£¬Čō¼ģŃé“ĖČÜŅŗÖŠŹĒ·ń“óĮæ“ęŌŚŅŌĻĀ6ÖÖĄė×Ó£ŗ¢ŁClO£ ¢ŚNH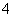 ¢ŪFe2£«¢ÜK£« ¢ŻHCO
¢ŪFe2£«¢ÜK£« ¢ŻHCO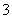 ¢ŽCl££¬ĘäÖŠ²»±Ų¼ģŃé¾ĶÄܼÓŅŌ·ń¶ØµÄĄė×ÓŹĒ(””””)
¢ŽCl££¬ĘäÖŠ²»±Ų¼ģŃé¾ĶÄܼÓŅŌ·ń¶ØµÄĄė×ÓŹĒ(””””)
A£®¢Ł¢Ś¢Ž”””””””””” ”” B£®¢Ś¢Ū¢Ü
C£®¢Ł¢Ū¢Ż D£®¢Ü¢Ż¢Ž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØÄÜŌŚĻĀĮŠČÜŅŗÖŠ“óĮæ¹²“ęµÄĄė×Ó×éŹĒ(””””)
¢Łŗ¬ÓŠ“óĮæAl3£«µÄČÜŅŗÖŠ£ŗNa£«”¢NH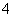 ”¢SO
”¢SO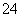 ”¢Cl£
”¢Cl£
¢Ś¼ÓČėAlÄܷųöH2µÄČÜŅŗÖŠ£ŗCl£”¢HCO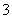 ”¢SO
ӢSO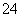 ӢNH
ӢNH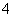
¢Ūŗ¬ÓŠ“óĮæFe3£«µÄČÜŅŗÖŠ£ŗNa£«”¢Mg2£«”¢NO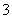 ”¢SCN£
”¢SCN£
¢ÜŌŚŗ¬ÓŠ“óĮæAlO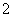 µÄČÜŅŗÖŠ£ŗNH
µÄČÜŅŗÖŠ£ŗNH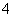 ”¢Na£«”¢Cl£”¢H£«
”¢Na£«”¢Cl£”¢H£«
¢ŻÓÉĖ®µēĄė³öµÄc(H£«)£½1”Į10£14mol”¤L£1µÄČÜŅŗÖŠ£ŗCa 2£«”¢K£«”¢Cl£”¢HCO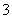
A£®¢Ł¢Ś B£®¢Ł¢Ū¢Ż
C£®¢Ł D£®¢Ł¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2012Äź3ŌĀ21 ČÕŹĒµŚ¶žŹ®½ģ”°ŹĄ½ēĖ®ČÕ”±£¬±£»¤Ė®×ŹŌ“£¬“ÓĪŅ×öĘš£”
(1)ClO2ŗĶCl2(»¹Ō²śĪļ¶¼ĪŖCl£)ŹĒÉś»īÖŠ³£ÓƵÄĻū¶¾¼Į”£µ±ĻūŗĵČĪļÖŹµÄĮæµÄĮ½ÖÖĪļÖŹŹ±£¬ClO2µÄĻū¶¾Š§ĀŹŹĒCl2µÄ________±¶”£
(2)ijĪŽÉ«·ĻĖ®ÖŠæÉÄÜŗ¬ÓŠFe3£«”¢Al3£«”¢Mg2£«”¢Na£«”¢NO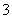 ”¢CO
ӢCO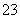 ӢSO
”¢SO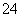 Ąė×ÓÖŠµÄ¼øÖÖ£¬ĪŖ·ÖĪöĘä³É·Ö£¬·Ö±šČ”·ĻĖ®ŃłĘ·100 mL£¬½ųŠŠĮĖČż×鏵Ń飬Ęä²Ł×÷ŗĶÓŠ¹ŲĻÖĻóČēĻĀĶ¼ĖłŹ¾£ŗ
Ąė×ÓÖŠµÄ¼øÖÖ£¬ĪŖ·ÖĪöĘä³É·Ö£¬·Ö±šČ”·ĻĖ®ŃłĘ·100 mL£¬½ųŠŠĮĖČż×鏵Ń飬Ęä²Ł×÷ŗĶÓŠ¹ŲĻÖĻóČēĻĀĶ¼ĖłŹ¾£ŗ
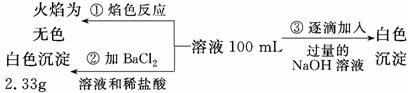
Ēėøł¾ŻÉĻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁŹµŃéÖŠŠčÅäÖĘ1.0 mol/LµÄNaOHČÜŅŗ100 mL£¬ĖłŠčŅĒĘ÷³żĮĖ²£Į§°ō ”¢ĶŠÅĢĢģĘ½”¢ĮæĶ²”¢Ņ©³×”¢ÉÕ±”¢½ŗĶ·µĪ¹Ü£¬»¹Č±ÉŁµÄŅĒĘ÷ĪŖ_________________________________________”£
”¢ĶŠÅĢĢģĘ½”¢ĮæĶ²”¢Ņ©³×”¢ÉÕ±”¢½ŗĶ·µĪ¹Ü£¬»¹Č±ÉŁµÄŅĒĘ÷ĪŖ_________________________________________”£
¢ŚŹµŃé¢ŪÖŠ³ĮµķĮæÓÉA”śB¹ż³ĢÖŠĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________”£
¢ŪŹŌČ·¶ØNO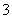 ŹĒ·ń“ęŌŚ£æ________(Ģī”°“ęŌŚ”±”¢”°²»“ęŌŚ”±»ņ”°²»Č·¶Ø”±)£¬Čō“ęŌŚ£¬ŹŌ¼ĘĖćc(NO
ŹĒ·ń“ęŌŚ£æ________(Ģī”°“ęŌŚ”±”¢”°²»“ęŌŚ”±»ņ”°²»Č·¶Ø”±)£¬Čō“ęŌŚ£¬ŹŌ¼ĘĖćc(NO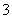 )£½________(Čō²»“ęŌŚ£¬“ĖĪŹ²»±Ų×÷“š)”£
)£½________(Čō²»“ęŌŚ£¬“ĖĪŹ²»±Ų×÷“š)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®·“Ó¦ŹĒ·ÅČČ»¹ŹĒĪüČȱŲŠėæ“Éś³ÉĪļŗĶ·“Ó¦ĪļĖł¾ßÓŠµÄ×ÜÄÜĮæµÄĻą¶Ō“óŠ”
B£®ĀĢÉ«Ö²Īļ½ųŠŠ¹āŗĻ×÷ÓĆŹ±£¬½«Ģ«ŃōÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ“¢“ęĘšĄ“
C£®ĪüČČ·“Ó¦ÖŠÓÉÓŚ·“Ó¦Īļ×ÜÄÜĮæŠ”ÓŚÉś³ÉĪļ×ÜÄÜĮ棬Ņņ¶ųĪŽĄūÓĆ¼ŪÖµ
D£®ĪļÖŹµÄ»ÆѧÄÜæÉŅŌŌŚŅ»¶ØĢõ¼žĻĀ×Ŗ»ÆĪŖČČÄÜ”¢µēÄÜĪŖČĖĄąĖłĄūÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆČēĶ¼×°ÖĆÖĘČ”±ķÖŠµÄĖÄÖÖøÉŌļ”¢“æ¾»µÄĘųĢå£ØĶ¼ÖŠĢś¼ÜĢØ”¢Ģś¼Š”¢¼ÓČČ¼°ĘųĢåŹÕ¼Æ×°ÖĆ¾łŅŃĀŌČ„£»±ŲŅŖŹ±æÉŅŌ¼ÓČČ£»a”¢b”¢c”¢d±ķŹ¾ĻąÓ¦ŅĒĘ÷ÖŠ¼ÓČėµÄŹŌ¼Į£©”£
ĘäÖŠÕżČ·µÄŹĒ£Ø £©
| Ń”Ļī | ĘųĢå | a | b | c | d |
| A | CO2 | ŃĪĖį | CaCO3 | ±„ŗĶNa2CO3 ČÜŅŗ | ÅØĮņĖį |
| B | Cl2 | ÅØŃĪĖį | MnO2 | NaOHČÜŅŗ | ÅØĮņĖį |
| C | NH3 | ±„ŗĶNH4Cl ČÜŅŗ | ĻūŹÆ»Ņ | H2O | ¹ĢĢåNaOH |
| D | NO | Ļ”ĻõĖį | ĶŠ¼ | H2O | ÅØĮņĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com