
���� ��1������m=CVM�������ʵ�������
��2�����ݲ�������ѡȡʵ��������
��3������ƿʹ��ǰ�ü���Ƿ�©ˮ��
��4����������һ�����ʵ���Ũ����Һһ�㲽����
��5�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��6������200mL0.01mol/LNaOH��50mL0.02mol/LCa��OH��2��Һ�к������������������ʵ���������C=$\frac{n}{V}$����Ũ�ȣ�
��� �⣺��1������0.1mol/L��NaOH��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ���ʵ�����=0.1mol/L��40g/mol��0.5L=2.0g��
�ʴ�Ϊ��2.0g��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ���ϴ��Һ��������ƿ����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�
����ʹ�õIJ������������ձ� ��500mL����ƿ �ݲ�������
����Ҫ�IJ������������ǣ���ͷ�ιܣ�
�ʴ�Ϊ���٢ܢݣ���ͷ�ιܣ�
��3������ƿʹ��ǰ�ü���Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4������һ�����ʵ���Ũ����Һ�IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����������ȷ��˳��Ϊ���ݢڢ٢ۢܣ�
��5����δϴ���ձ������������������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʲ�ѡ��
�ڳ���NaOH��ʱ��̫�������³�ȡ���������Ƶ����ʵ���ƫ�ͣ���ҺŨ��ƫ�ͣ��ʲ�ѡ��
�۶���ʱ���ӿ̶ȣ�������Һ���ƫС����ҺŨ��ƫ��ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
��NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ����ȴ����Һ�����С����ҺŨ��ƫ��ѡ��
��ѡ���ۢݣ�
��6��200mL0.01mol/LNaOH��50mL0.02mol/LCa��OH��2��Һ��n��OH-��=0.2L��0.01mol/L+0.02mol/L��2��0.05L=0.004mol��
C=$\frac{0.004mol}{0.25L}$=0.016mol/L��
�ʴ�Ϊ��0.016��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע���������ķ�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
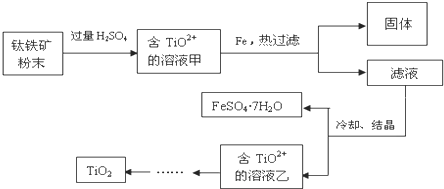
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | V3��V2��V1 | B�� | V1=V2=V3 | C�� | V2��V1=V3 | D�� | V1=V2��V3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͭ����������Һ��Ӧ��Cu+Ag+�TCu2++Ag | |
| B�� | ����������ʵ���Ũ�ȵ�����������Һ��̼�������Һ���Ba2++2OH-+NH4++HCO3-�TBaCO3��+NH3•H2O+H2O | |
| C�� | ��Ca��ClO��2��Һ��ͨ���������Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO | |
| D�� | �Ȼ�����Һ��ͨ�������̼���壺Ca2++CO2+H2O�TCaCO3��+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ��Ӧ���¶ȼ�ˮ��������������ƿ֧�ܿڴ� | |
| B�� | ����ʱ�������ˮ�����˿̶��ߣ�����ý�ͷ�ι��������ಿ�� | |
| C�� | ����ϡ����ʱ�������ձ��м���һ����ˮ�����ز�������������Ũ���� | |
| D�� | ����NaOHʱ��NaOH����С�ձ��з���������ƽ���̣������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ����� | ʵ��Ŀ�� | |
| A | ������ˮ����м����ڴ������ܵ���ƿ�� | �Ʊ��屽 |
| B | ������Һˮ�����ȴ�����£��ӵ�ˮ�۲����� | ��������Ƿ���ȫˮ�� |
| C | ��������Һ�м�������ϡ���ᣬˮԡ���ȣ��ټ������Ƶ�Cu��OH��2������������ | ��������ˮ����� |
| D | ������������������ˮ��Һ����һ��ʱ�䣬������ȴ��Ļ��Һ�еμ���������Һ | �����������е���ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
 ��
��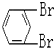
 ��
��
 ��
��
 ��CH3-CH2Cl��
��CH3-CH2Cl���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com