
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
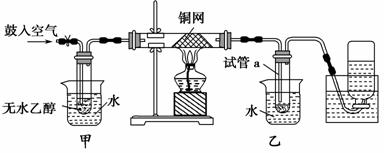
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��_____________________________________________________________
________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
(2)��������ˮԡ�����ò���ͬ��
��������________���ҵ�������___________________________________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������________________________________________________________________��
����ƿ���ռ������������Ҫ�ɷ���________________________________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b����
c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
������(1)���Ҵ��Ĵ�����ʵ���У�Cu����������Ӧ�����У���ɫ��Cu�����ɺ�ɫ��CuO����ɫ��CuO�ֱ��Ҵ���ԭΪ��ɫ��Cu���йصĻ�ѧ����ʽΪ2Cu��O2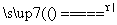 2CuO��CuO��CH3CH2OH
2CuO��CuO��CH3CH2OH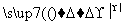 Cu��CH3CHO��H2O��
Cu��CH3CHO��H2O��
(2)��ˮԡ���ȵ�Ŀ���ǻ��ƽ�ȵ��Ҵ�����������ˮԡ��Ŀ����Ϊ��������ȩ��
(3)���ɵ�CH3CHO��H2O�Լ��ӷ��������Ҵ������Թ�a�������ռ���������ˮ��N2���ռ��ڼ���ƿ�С�
(4)��ʹ��ɫʯ����ֽ�Ժ�ɫ��˵����Һ��Ϊ�������ʣ���CH3COOH��Ҫ��ȥ��ȩ�е����ᣬ�����Ƚ�����NaHCO3��Ӧ����CH3COONa���ټ�����������CH3CHO��
�𰸡�(1)2Cu��O2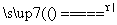 2CuO��
2CuO��
CH3CH2OH��CuO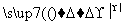 CH3CHO��H2O��Cu������
CH3CHO��H2O��Cu������
(2)���ȡ���ȴ��(3)��ȩ���Ҵ���ˮ������
(4)���ᡡc������


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�輰�仯����������ִ�������������ף���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����������ͨ�������ݹ�̫���ܵ��
A���٢ڢ� B���ۢܢ�
C���ڢۢ� D���٢ۢ�
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6�棬�ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���S iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
(4)��ҵ�Ͽ���SiCl4(g)�Ʊ����½ṹ�մɵ����裬�䷴Ӧ����ʽΪ
 3SiCl4(g)��2N2(g)��6H2(g)
3SiCl4(g)��2N2(g)��6H2(g) Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
�ٸ÷�Ӧ��ƽ�ⳣ������ʽK��______________.
�����ܱպ��������У��ܱ�ʾ������Ӧ�ﵽƽ��״̬����_______ _��
_��
A��3v��(N2)��v��(H2)
B��v��(HCl��4v����4v��(SiCl4)
C����������ܶȱ��ֲ���
D��c(N2)��c(H2)��c(HCl)��1��3��6
����ij�����´ﵽƽ��ʱ��H2��HCl���ʵ���֮��Ϊm��n�����������������䣬�����¶ȴﵽƽ��ʱ��H2��HCl���ʵ���֮��________m��n(�>��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
300����ǰ��������ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����Ľṹ��ʽΪ��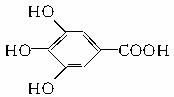
(1)��ûʳ��������īˮ��Ҫ������________����������(�����)��
A���� B���� C����֬ D������
(2)ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ________________________________________________________________��
(3)�Ჴ�����Ƕ��ǻ��������봼�γɵ��������ǹ�������ʹ�õ�ʳƷ���Ӽ����Ჴ�����ķ���ʽΪ________���䱽��ֻ�롪OH�͡�COOR����ȡ����ֱ��������ͬ���칹����________�֡�
(4)д���Ჴ������������������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ��_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�

��֪��R��CH===CH2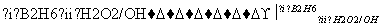 RCH2CH2OH(����B2H6Ϊ������)
RCH2CH2OH(����B2H6������)
��ش��������⣺
(1)11.2 L(��״��)����A�������г��ȼ�տ�������88 g CO2��45 g H2O��A�ķ���ʽ��________________________________________��
(2)B��C��Ϊһ�ȴ��������ǵ�����(ϵͳ����)�ֱ�Ϊ___________��
(3)�ڴ���������1 mol F��2 mol H2��Ӧ������3����1������F�Ľṹ��ʽ��___________________________________��
(4)��Ӧ�ٵķ�Ӧ������_____________________________��
(5)��Ӧ�ڵĻ�ѧ����ʽΪ__________________________��
(6)��G������ͬ�����ŵ�G�ķ�����ͬ���칹�干�����֣��������ֱַ���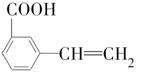 ��
�� ���������ֵĽṹ��ʽ�ֱ���______________
���������ֵĽṹ��ʽ�ֱ���______________
_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У����ڴ��ǻ���ȡ������ (���� )
A���Ҵ��ͽ����Ƶķ�Ӧ
B���Ҵ�������ķ�Ӧ
C�����Ҵ�����ϩ�ķ�Ӧ
D���Ҵ���Ũ��������Һ�ķ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҿ��þƾ���Ũ�������Լ�����ȡ��ϩ����ʵ��������������ั��Ӧ�������練Ӧ�л�����SO2��CO2��ˮ���������ij�о���ѧϰС��������ͼ��ʾ��װ���Ʊ���������ϩ��̽����ϩ�뵥�����ܷ�Ӧ����Ӧ���͡��ش��������⣺
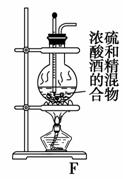
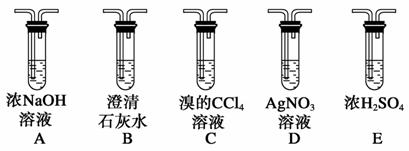
(1)д���Ʊ���ϩ��Ӧ�Ļ�ѧ����ʽ��__________________________________
________________________________________________________________��
ʵ���У����Ũ�������Ҵ��ķ����ǽ�______________����������һ�������У�����Fװ��ʱ����ʹҺ���¶�__________________��
(2)д��Ũ������ƾ�ֱ�ӷ�Ӧ����������������Ļ�ѧ����ʽ�� ________________________________________________________________��
(3)Ϊʵ������ʵ��Ŀ�ģ�װ�õ�����˳��ΪF��__________________��D��(��װ������һ��)
(4)��C�й۲쵽____________ʱ������������������ϩ��Ӧ����D��____________ʱ������C�з�������ȡ����Ӧ����Dû�г���ǰ��������������ʱ������C�з�������________��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ�IJ�����ʵ����������֤���ӵ��������ʡ���
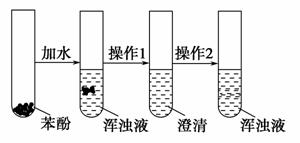
(1)���ʢ���__________������1��________������2��________��
(2)���ʢ���__________������1��________������2��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȩ����Ũ���������㣬��ṹ��ʽ����ͼ��ʾ��

��������ȩ�����������������
(����)
A���ڼ��Ⱥʹ��������£��ܱ�������ԭ
B���ܱ����Ը��������Һ����
C����һ�������������巢��ȡ����Ӧ
D�������������ᷢ���ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�������ֵ������������ȷ����
A��1molFeCl3����ˮ��Ӧת��������������������ɽ���������ĿΪNA
B����⾫��ͭʱ����ת����NA�����ӣ�����������32 gͭ
C��6.8�����ڵ�KHSO4���к���0.1NA��������
D����״���£�11.2L���Ȼ�̼����������Ϊ0.5NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com