
�ڻ���ƽ�����װ��ǿ��ԭ����(N2H4Һ̬)��������H2O2�������ǻ��ʱ����������N2��ˮ�������ų������ȡ���֪0.4 mol N2H4(l)������H2O2(l)��Ӧ����N2��ˮ�����ų�256.65 kJ��������
(1)д����Ӧ���Ȼ�ѧ����ʽ___________________________��
(2)��֪H2O(g)===H2O(l)����H����44 kJ·mol��1����16 g Һ̬��������H2O2(l)��Ӧ����N2��Һ̬ˮʱ���ų�����______________kJ��
(3)������Ӧ���ڻ���ƽ��������ͷų������ȺͿ��ٲ������������⣬����һ����ͻ�����ص���________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��ͼΪ���ѵ�أ�����˵����ȷ����(����)
A��һ��ʱ���пƬ�������С
B��ͭ�缫�����������ɫ
C��������ͭͨ����������п
D��п�缫�Ǹõ�ص�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������X����Һ�ֱ����ʢ��10 mL 2 mol/L������ձ��У�������ˮϡ�͵�50 mL����ʱX��������з�Ӧ�����з�Ӧ����������(����)
A��20 mL��3 mol/L B��25 mL��2 mol/L
C��10 mL��4 mol/L D��10 mL��2 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾij���巴Ӧ�������仯ʾ��ͼ���ݴ˷��������жϴ������(����)

A������һ�����ȷ�Ӧ
B���÷�Ӧ������Ҫ����
C������������������ڷ�Ӧ���������
D����Ӧ�����������ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�Ħ�Hǰ�ߴ��ں��ߵ���(����)
��C(s)��O2(g)===CO2(g)����H1
C(s)�� O2(g)===CO(g)����H2
O2(g)===CO(g)����H2
��S(s)��O2(g)===SO2(g)����H3
S(g)��O2(g)===SO2(g)����H4
��H2(g)�� O2(g)===H2O(l)����H5
O2(g)===H2O(l)����H5
2H2(g)��O2(g)===2H2O(l)����H6
��CaCO3(s)===CaO(s)��CO2(g)����H7
CaO(s)��H2O(l)===Ca(OH)2(s)����H8
A���� B����
C���ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����M��N��P��E 4��Ԫ�صĵ��ʣ��ܷ������·�Ӧ��
����ˮ��Һ�У�M��N2��===M2����N����P��2H2O(��)===P(OH)2��H2������N��E��������ϡH2SO4�У��缫��ӦΪ��N��2e��===N2����2H����2e��===H2�����ж����ǵĻ�ԭ����ǿ������˳����(����)
A��M��N��P��E B��P��M��N��E
C��M��N��E��P D��E��P��M��N
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ������¼���£�
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Al��Mg | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C(ʯī) | ϡ���� | ƫ��ʯī |
| 4 | Al��Mg | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�Ը����ϱ��е�ʵ������ش��������⣺
(1)ʵ��1��2��Al�����ĵ缫(������)�Ƿ���ͬ(��ǡ���)��________��
(2)��ʵ��3���������գ�
����Ϊ________�����缫��Ӧʽ��______________________��
��ʯīΪ________�����缫��Ӧʽ��_____________________��
�۵���ܷ�Ӧʽ��_____________________________________��
(3)ʵ��4������������������________��������______________________________��д�����缫�ĵ缫��Ӧʽ��__________________________________________________��
(4)����ʵ��5�е�����ָ��ƫ������ԭ��__________________________________________________��
(5)����ʵ�����ܽ��Ӱ������ԭ������������������أ�____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɫ��ѧ�ᳫ��������Ӧ���ԭ�������ʣ�ԭ�������ʱ�ʾĿ������������������������֮�ȡ��������Ʊ���������ķ�Ӧ�У�ԭ����������ߵ���(����)
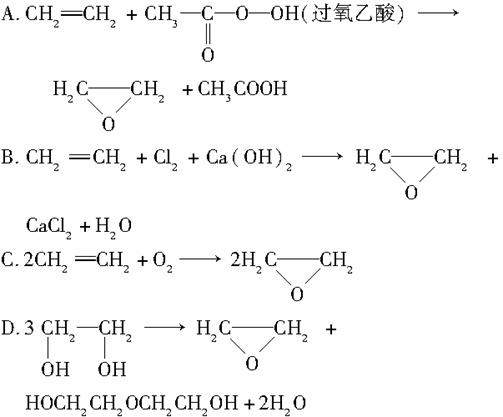
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ܱ������ڼ���a mol PH4I���壬��һ���¶��·������·�Ӧ
PH4I��S��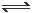 PH3��g�� + HI��g�� �� ��
PH3��g�� + HI��g�� �� ��
4PH3��g�� 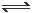 P4��g��+ 6 H2��g�� �� ��
P4��g��+ 6 H2��g�� �� ��
2HI��g��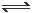 H2��g�� + I2��g�� ��
H2��g�� + I2��g�� ��
��������Ӧ����ƽ����HIΪb mol��I2(��)Ϊc mol��H2Ϊd mol����
�� ƽ���������P4(��)��PH3�����ʵ������ô���ʽ��ʾ����n(P4)= ��n(PH3)= ��
�� a��b��c���ߵĹ�ϵ����a > (�b��c�Ĵ���ʽ)��
�� ƽ�������ѹǿ��������n(I2) , n(PH4I) (���ӡ����١�����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com