
| A | B | C | |
| ��ʼʱ�Ͻ��ĩ������/mg | 204 | 399 | 561 |
| ��Ӧ����ʱ������������/mL | 224 | 336 | 336 |
| A�� | ��ʼʱ�����Ũ��Ϊ1mol•L-1 | B�� | �����NaOH��ҺΪ40mL | ||
| C�� | �Ͻ���þ���������ʵ������ | D�� | ���õ���Һ�к����������� |
���� ����Ũ�ȡ����һ����A�кϽ�����С��B�кϽ���������A�������������С��B�����������˵��A�����������������ȫ��Ӧ��B�кϽ�����С��C�кϽ���������B��C�������������ȣ�˵��B��C��������ȫ��Ӧ������336mL������Ҫ����������Ϊ��204mg��$\frac{336mL}{224mL}$=306mg��399mg����B�н���ʣ�࣬����㣮
A��B��C��������ȫ������n=$\frac{V}{{V}_{m}}$�������������ʵ�����������Ԫ���غ��֪n��HCl��=2n��H2��������������������ʵ���Ũ�ȣ�
B��A��������ʣ�࣬������ȫ��Ӧ����ʱ��������2224mL���ʿ��Ը���A�����ݼ�����������ʵ�������þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼���x��y��ֵ����������C��Al�����ʵ�����
��C�ձ��м���һ������1mol/L��NaOH��Һ��ʹ�Ͻ��е�����ǡ����ȫ�ܽ⣬ȴ���ܺ����ij�������ʹMg2+�պó�����ȫ����Һ������ΪNaCl��NaAlO2�������������غ��֪n��NaOH��=n��NaCl��+n��NaAlO2�����ݴ˼����������Ƶ����ʵ������ٸ���V=$\frac{n}{c}$�����������Ƶ������
C������B�м����֪Al��Mg���ʵ�����ϵ��
D����Һ������ΪNaCl��NaAlO2��
��� �⣺����Ũ�ȡ����һ����A�кϽ�����С��B�кϽ���������A�������������С��B�����������˵��A�����������������ȫ��Ӧ��B�кϽ�����С��C�кϽ���������B��C�������������ȣ�˵��B��C��������ȫ��Ӧ������336mL������Ҫ����������Ϊ��204mg��$\frac{336mL}{224mL}$=306mg��399mg����B�н���ʣ�࣬����㣮
A��B��C��������ȫ���������������ʵ���Ϊ$\frac{0.336L}{22.4L/mol}$=0.015mol��������Ԫ���غ��֪n��HCl��=2n��H2��=0.03mol������������ʵ���Ũ��Ϊ$\frac{0.03mol}{0.03L}$=1mol/L����A��ȷ��
B����A��Mg�����ʵ���Ϊx��Al�����ʵ���Ϊy��������������ʵ���Ϊ��$\frac{0.224L}{22.4L/mol}$=0.01mol��
�ɺϽ�������ɵã�24x+27y=0.204��
���ݵ���ת���غ�ɵã�2x+3y=0.01��2
�������̣���ã�x=0.004mol��y=0.004mol��
��֪C��Al��Mg�����ʵ�����Ϊ0.004mol��$\frac{561g}{204g}$=0.011mol��
��C�ձ��м���һ������1mol/L��NaOH��Һ��ʹ�Ͻ��е�����ǡ����ȫ�ܽ⣬ȴ���ܺ����ij�������ʹMg2+�պó�����ȫ����Һ������ΪNaCl��NaAlO2�������������غ��֪n��NaOH��=n��NaCl��+n��NaAlO2��=
0.03mol+0.011mol=0.041mol�������NaOH��Һ�����Ϊ$\frac{0.041mol}{1mol/L}$=0.041L=41mL����B����
C������B�м����֪Al��Mg���ʵ�����ȣ���C��ȷ��
D����Һ������ΪNaCl��NaAlO2����Һ�к���������ΪCl-��AlO2-��OH-����D��ȷ��
��ѡ��B��
���� ���⿼������ļ��㣬��Ŀ�ѶȽϴ��ݱ��������ж����ʹ�������ǽ���Ĺؼ������ؿ���ѧ�������ݵķ������������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
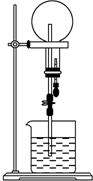 ��֪NH3��HCl��������������Ȫʵ������壮����ͬ��ͬѹ���õ������ƿһ���ռ���NH3����һ���ռ�HCl��N2�Ļ�����壬��ͼ��ʾ����Ȫʵ��ֹͣ��������ƿ����Һ�Ĺ�ϵ�ǣ���������ƿ�����ʵ���ɢ����ˮ�����ʰ�NH3���㣩��������
��֪NH3��HCl��������������Ȫʵ������壮����ͬ��ͬѹ���õ������ƿһ���ռ���NH3����һ���ռ�HCl��N2�Ļ�����壬��ͼ��ʾ����Ȫʵ��ֹͣ��������ƿ����Һ�Ĺ�ϵ�ǣ���������ƿ�����ʵ���ɢ����ˮ�����ʰ�NH3���㣩��������| A�� | ���ʵ����ʵ���Ũ����ͬ�����ʵ�����������ͬ | |
| B�� | ���ʵ�����������ͬ�����ʵ����ʵ���Ũ�Ȳ�ͬ | |
| C�� | ���ʵ����ʵ���Ũ�Ⱥ����ʵ�������������ͬ | |
| D�� | ���ʵ����ʵ���Ũ�Ⱥ����ʵ�������������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ƿ��Ǵ������ӻ����ӵļ����� | B�� | ��ɢ����ֱ���Ĵ�С��ͬ | ||
| C�� | �Ƿ��ж����ЧӦ | D�� | �Ƿ��һ���ȶ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
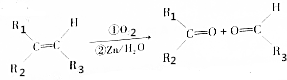
 ��
�� ��1mol��
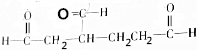
��1mol��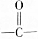 ������������������ԭ�Ӹ�����ȵ�Ԫ����C��H��
������������������ԭ�Ӹ�����ȵ�Ԫ����C��H�� �ɼ�дΪ
�ɼ�дΪ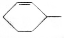 �������߱�ʾ��ѧ�����ߵĶ˵㡢�۵���ʾ̼ԭ�ӣ�̼ԭ��ʣ��Ļ��ϼ�����ԭ�Ӳ��㣮д��A���п��ܵĽṹ��ʽ��
�������߱�ʾ��ѧ�����ߵĶ˵㡢�۵���ʾ̼ԭ�ӣ�̼ԭ��ʣ��Ļ��ϼ�����ԭ�Ӳ��㣮д��A���п��ܵĽṹ��ʽ��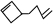 ��
��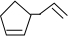 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2 CH2CH3 | B�� | CH3COOCH2CH3 | C�� | CH3CH2COOH | D�� | CH3CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƹ㡰��̼���á�����������������ŷ� | |
| B�� | ������չ����Դ����������������ŷ� | |
| C�� | �ƹ㡰��ɫ���ɡ��ƻ������տ����е�CO2��������Դ�ϳ����� | |
| D�� | �ƹ�С��������վ���˽�������ط��õ����ѣ��ٽ��ط����õĿ��ٷ�չ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com