
下列操作不能达到目的的是( )
选项目的操作
A.配制100 mL 1.0 mol·L-1 CuSO4溶液将25 g CuSO4·5H2O溶于100 mL蒸馏水中
B.除去KNO3中少量NaCl将混合物制成热的饱和溶液,冷却结晶,过滤
C.提取溴水中的Br2向溶液中加入乙醇后振荡,静置,分液
D.溶液中是否含有NH4+ 取少量溶液于试管中,加入NaOH后,加热,在试管口放置一块湿润的石蕊试纸
 高中必刷题系列答案
高中必刷题系列答案科目:高中化学 来源:2016届江苏省宿迁市高一上学期第二次月考化学试卷(解析版) 题型:选择题
下列有关试剂的保存方法,错误的是
A.金属钠保存在煤油中
B.存放FeSO4溶液时加入少量铁粉
C.NaOH溶液保存在带胶塞的玻璃试剂瓶中
D.新制的氯水保存在无色广口瓶中
查看答案和解析>>
科目:高中化学 来源:2016届江苏省南京市高淳区高一上学期期末考试化学试卷(解析版) 题型:选择题
下列各组中的离子,能在溶液中大量共存的是
A.Na+、Cu2+、Cl-、SO42- B.Na+、Ca2+、CO32-、NO3-
C.Na+、H+、Cl-、CO32- D.K+、H+、SO42-、OH-
查看答案和解析>>
科目:高中化学 来源:2016届江苏省南京市高淳区高一上学期期末考试化学试卷(解析版) 题型:选择题
新年伊始,我国中东部各地陆续出现大范围和长时间雾霾天气,主要原因是由于大气中PM2.5含量升高所造成,PM2.5是指大气中直径小于或等于2.5微米(1微米=10-6米)的颗粒物。其中PM2.5的主要来源与汽车排放的尾气有关。下列有关叙述正确的是
A.胶体的本质特征是具有丁达尔现象
B.将直径等于2.5微米的颗粒物分散于水即成为胶体
C.雾具有丁达尔现象
D.增加使用汽车,提高交通效率可缓解雾霾天气的产生
查看答案和解析>>
科目:高中化学 来源:2016届江苏省东台市高一上学期期末考试化学试卷(解析版) 题型:填空题
有关物质间转化关系如下图,试回答:
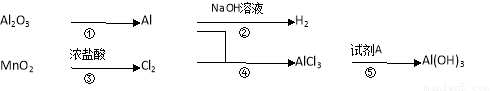
(1)转化①的反应条件为 .
(2)转化②的化学方程式为 .转化③的离子方程式为 .
(3)若转化⑤用于实验室制Al(OH)3,则试剂A宜选择 (填名称).
(4)向50 mL 3 mol·L-1 AlCl3溶液中滴加1 mol·L-1 NaOH溶液,结果Al3+离子有1/3转化为Al(OH) 3沉淀,则加入的NaOH溶液的体积可能为 或 mL。
查看答案和解析>>
科目:高中化学 来源:2016届江苏省东台市高一上学期期末考试化学试卷(解析版) 题型:选择题
配制250 mL 0.10 mol/L的NaOH溶液时,下列实验操作会使配得的溶液浓度偏小的是 ( )
A.容量瓶未干燥就直接加水定容
B.在容量瓶中进行定容时仰视刻度线
C.称量的NaOH固体已潮解
D.定容后把容量瓶倒转摇匀,发现液面低于刻度
查看答案和解析>>
科目:高中化学 来源:2016届江苏省东台市高一上学期期末考试化学试卷(解析版) 题型:选择题
下列叙述中错误的是( )
A.Cl2可用于自来水的杀菌消毒 B.常温下,铝制容器盛放稀硫酸
C.Al2O3性质用作耐火材料 D.硅酸钠用作木材的防火剂
查看答案和解析>>
科目:高中化学 来源:2016届江苏南京市高一上学期期中考试化学试卷(解析版) 题型:选择题
某溶液中存在较多的H+、SO42-、C1-,该溶液中还可能大量存在的离子是
A.OH- B.Ba2+ C.NH4+ D.Ag+
查看答案和解析>>
科目:高中化学 来源:2016届广东省梅州市高一上学期质检化学试卷(解析版) 题型:选择题
元素R、X、T、Z、Q在元素周期表中的相对位置如下表所示, 其中R单质在暗处与H2剧烈化合并发生爆炸。则下列判断正确的是
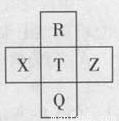
A.非金属性:Z<T<X
B.R与Q的电子数相差26
C.气态氢化物稳定性:R <T<Q
D.最高价氧化物的水化物的酸性:T>Q
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com